Citation: Manser HP, Egloff C, King MC, “Large-Volume Wearable Drug Delivery: a Vision Becomes Reality”. ONdrugDelivery Magazine, Issue 100 (Sep 2019), pp 74-76.
Hans Peter Manser, Christoph Egloff and Martin C King introduce LV DDS , a flexible-form, low-profile wearable injector platform, for large-volume drug delivery.
Pharmaceutical companies around the globe are focusing on simplified, affordable, large-volume delivery of parenteral drugs. There is a strong trend for subcutaneous delivery and self-administration that is leading to even larger injection volumes and higher viscosity formulations.

Figure 1: The Weibel CDS LV DDS wearable injector.
Weibel CDS recently delivered the first prefilled customer units of a new low-profile (22.3 mm) large-volume (25 mL) wearable injector with a flexible form drug reservoir (Figures 1 and 2). It is a single-use disposable device that is worn attached directly to the skin with an integrated fluid path, automatic needle insertion, soft cannula placement and infusion/injection system. The drug reservoir is capable of containing volumes up to 50 mL.
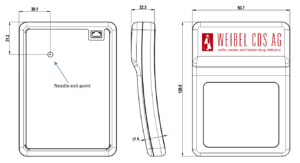
Figure 2: Example based on 25mL and fully disposable LV DDS.
The units were the first from Weibel CDS’s new Large-Volume Drug Delivery System (LV DDS) platform, based on proven Weibel CDS innovations:
- The Drug Delivery System, DDS, a valveless volumetric continuous micro-displacement pump
- The MiniBag, a flexible form primary container with similar drug contact properties to glass.
LARGE-VOLUME DRUG DELIVERY: SIMPLIFIED
User-filled or prefilled, the single-use Weibel CDS LV DDS simplifies drug administration at home, or in the clinical environment by healthcare professionals, to three steps:
- Peel-off backing
- Attach device to body
- Start injection.
Ready for Customisation
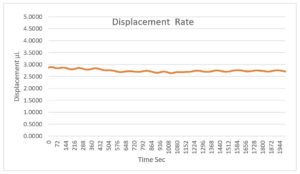
Figure 3: The pump has a constant displacement rate in compliance with IEC 60601-2-24.
The LV DDS platform is ready for customisation to specific “intended-use” user- or drug product-specific requirements.
With unsurpassed dose accuracy (Figure 3), its continuous volumetric displacement pump (Figure 4) supports a range of high viscosity large molecule drug products.
The Weibel CDS LV DDS supports the following features:
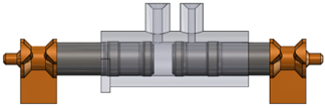
Figure 4: Valveless volumetric displacement pump.
- Safe, user-friendly simple to use system with minimised steps.
- Automatic needle insertion system (ANIS), with all needle safety steps performed automatically. The needle remains hidden at all times and is made safe after injection and device removal.
- Automatic soft cannula placement as an option for long duration injections and patient comfort.
- Automatic injection commences when attached to the skin and the drug is ready for injection.
- Suitable for combination therapies from a single device.
- Suitable for continuous flow slow injection, bolus and long duration injections
- Unique high dose accuracy, continuous volumetric pump supporting a range of high viscosity drug products.
- Programmable electronics provide:
– Personalised patient feedback and connectivity
– “Patient-personalised” programmable injection time and rate profile for the healthcare professional.
- Customisable device shell supporting customer-specific branding schemes.
- Preloaded or loaded at time of use without the need of a cleanroom environment.
The key parameters and technical specs of the LV DDS are summarised in Table 1.
| Min. | Typ. | Max. | Units | |
| Water Proofing | IP 33 | |||
| Device Weight (Empty Reservoir) | 100 | g | ||
| Operating Temperature | +4 | +23 | +37 | °C |
| Operating relative humidity range | 20 | 90 | % | |
| Non-condensing Operating atmospheric pressure |
600 | 1065 | hPA | |
| Viscosity of forwarded Medium | 100+ | cP | ||
| Reservoir | 40 | mL | ||
| Dosage Range with liquid viscosity of 10cP |
tbd | 360,000 | μL/h | |
| Single Stroke Pump Volume | 10 | 200 | μL |
+Depending on the drug delivery rate required, higher viscosities may be possible.
Table 1: Key parameters and technical specs of the Weibel CDS LV-DDS.
Low-Shear-Force Volumetric Displacement Pump
The Weibel CDS valveless volumetric displacement pump (Figure 3) performs well with highly viscous products and offers good compatibility with shear force-sensitive drugs resulting in no measurable change to the protein structure. Weibel CDS provides Test Platforms for Pharmaceutical Companies to evaluate the delivery characteristics of drug products in the security of their own laboratories.
MiniBagSystem

Figure 5: MiniBagSystem is a primary packaging solution with similar drug contact properties to glass.
MiniBagSystem (Figure 5) has undergone rigorous mechanical characterisation and stability testing with large-molecule drug products confirming it as a primary packaging solution with similar drug contact properties to glass, and resilience to pharmaceutical industry standard sterilisation processes.
Exclusive to Weibel CDS, this cyclic olefin copolymer/polychlorotrifluoroethylene (COC/PCTFE) flexible film, CETA160, has been specially developed to store drug product. Manufactured to cGMP standards, the film is high-barrier, transparent, radiation sterilisation stable, non-yellowing and US FDA-compliant.
| Water vapour transmission rate (@38°C, 90% RH) | 0.06 g / m² / 24 h | 0.004 g / 100 in² / 24 h |
| Oxygen transmission rate (@23°C, 50% RH) | 19 cm³ / m² / 24 h | 1.23 cm³ / 100 in² / 24 h |
Table 2: Barrier properties of Aclar® film used for the MiniBagSystem.
The PCTFE element is Aclar® from Honeywell International, which gives the film its high barrier to moisture and to aromatic and aliphatic hydrocarbon species and excipients (Table 2).

