Citation: Billard F, Mao L, “Spray Characterisation Testing for Orally Inhaled and Nasal Drug Products”. ONdrugDelivery, Issue 119 (Apr-May 2021), pp 52–55.
Francois Billard and Lei Mao look at measurement systems for spray pattern and plume geometry dispensed by orally inhaled and nasal drug products.
BACKGROUND
“SprayVIEW® measurement systems from Proveris Scientific are the industry standard for the measurement of SP and PG dispensed by nasal sprays, oral sprays and MDIs.”
Inhalation products are extremely complex to develop and manufacture, and it is important to understand potential interactions between the formulation and the delivery device throughout the development stages. For the characterisation of orally inhaled and nasal drug products (OINDPs) including nasal sprays, oral sprays and metered-dose inhalers (MDIs), spray performance, such as droplet size distribution, spray pattern (SP) and plume geometry (PG) can be assessed using laser-based techniques. These techniques include the use of specific mechanical equipment for the actuation of the device, which generates the spray, combined with laser-based equipment for the characterisation of the spray.
SprayVIEW® measurement systems from Proveris Scientific are the industry standard for the measurement of SP and PG dispensed by nasal sprays, oral sprays and MDIs. They are used by pharmaceutical companies, contract development and manufacturing organisations (CDMOs) and contract research organisations, as well as manufacturers of metered valves and pumps, and regulatory agencies worldwide.
For device actuation, the use of automated equipment such as Vereo® Actuator NSx (Proveris) (for nasal and oral sprays) or Vereo® Actuator MDx (Proveris) (for MDIs) is required to eliminate the variability associated with manual actuation. The automated actuation stations of SprayVIEW® measurement systems allow for consistent actuation of the device. Motion is controlled via an electromechanical motor that requires input parameters such as stroke length, velocity, acceleration and hold time. These systems allow for controlled and repeatable actuations and, as such, they are ideal for quality control release testing, and they also offer valuable support for product development to assess the overall performance of the product throughout its lifecycle.
For spray characterisation, real-time images of aerosols or sprays emitted from the device through a laser sheet are acquired using a high-speed camera. For SP measurements, the laser sheet is positioned in a direction perpendicular to the axis of the spray at a given distance from the device tip. For PG measurements, the laser sheet is orientated in the axis of the spray through the device tip, and the plume is imaged from the side. Subsequent data analysis of SP (via a cross section of the aerosol or spray perpendicular to the axis of the plume) and PG (via a side view of the aerosol or spray parallel to the axis of the plume) can be used to determine the shape and size of the aerosol or spray.
SP and PG tests are required for chemistry, manufacturing and controls (CMC) characterisation by the US FDA. SP analysis must be included in the release specification for the finished drug product and, for MDIs, must appear in stability studies of registration batches. PG, on the other hand, is complementary to SP. It does not have to be tested on a routine basis and so does not have to be included in the release specification for the finished drug product. However, as with SP, it must be included in the stability studies of registration batches for MDIs.
DETERMINATION OF ACTUATION PARAMETERS
Actuation parameters are typically the first settings that should be established when developing SP and PG methods for OINDPs. The FDA recommends that actuation settings should reflect proper usage of the product by trained patients and be documented based on exploratory studies in which the relevant parameters are varied to simulate in vitro performance upon hand actuation.1 Such actuation settings may be provided by the suppliers of metered valves and pumps to be used as a starting point for product development.
In the absence of recommendations from the pump supplier, human actuation studies can also be provided by Proveris Scientific®. Using the company’s proprietary Ergo™ technology, hand actuation data are acquired from trained testers in the targeted population for the product, and through statistical analysis the information is translated into average values and ranges for the input parameters of the equipment, including stroke length, velocity, acceleration and hold time. These settings will enable the automated actuator to operate and produce actuations based on actual data from human actuations.
METHOD DEVELOPMENT
SP testing is typically performed on a routine basis as a quality control method for release of the drug product, while PG testing is only performed during the characterisation of the product. SP testing is used to report qualitative metrics such as shape (e.g. circular, ellipsoid) and quantitative metrics such as size (e.g. longest diameter [Dmax], shortest diameter [Dmin] and ratio of Dmax/Dmin), which should lie in a specified range (for in vitro population bioequivalence [PBE] studies, area will also need to be assessed). PG testing is used to report metrics such as plume angle and width reported at a single delay time while the fully developed phase of the plume is still in contact with the actuator tip.
Once the actuation settings have been established, the parameters to be optimised during method development will typically include camera position, tip-to-laser distance, frame rate (125, 250 or 500 frames per second), as well as spray duration (start and stop time for analysis) in the case of SP, or time delay (single frame representative of the stable phase of the plume) in the case of PG.
The overall goal in method development is to identify the optimum operating parameters where test results will be most repeatable. Points that need to be considered during method development include:
- For SP, camera placement should be optimised so that SPs are consistently within the field of view. It is recommended that the SP percentage area be at least 5% of the total field of view. However, it is important to keep in mind that, although higher SP percentage area values will increase the size of the pattern and will improve overall measurement sensibility, it could also lead to patterns outside the field of view and unable to be measured if the patterns are too large.
- Similarly, for PG testing, it should be ensured that the edges of the plume are consistently within the field of view. The measurement of wide plumes may require the use of a wide-angle lens.
- There is a risk of the device being detected by the camera, which can result in the software of the SprayVIEW® measurement system processing the image of the device as a second pattern. This phenomenon is caused by light reflection from the laser on the device. Whenever this occurs, reflection light blockers should be used.
- All sources of variability should be thoroughly assessed during method development. It should not be assumed that measurement variability is inherent to the method or solely due to interactions between device and formulation. For instance, it should be ensured that the actuation of the device is robust. The device must be securely connected to the actuation station using the appropriate jig to avoid any movement during firing, as this could cause some measurement variability. The design of the jig should also ensure proper alignment of the device. The test environment could be another source of measurement variability. For instance, formulations containing ethanol may exhibit variability in spray performance depending on the relative humidity level in the laboratory.
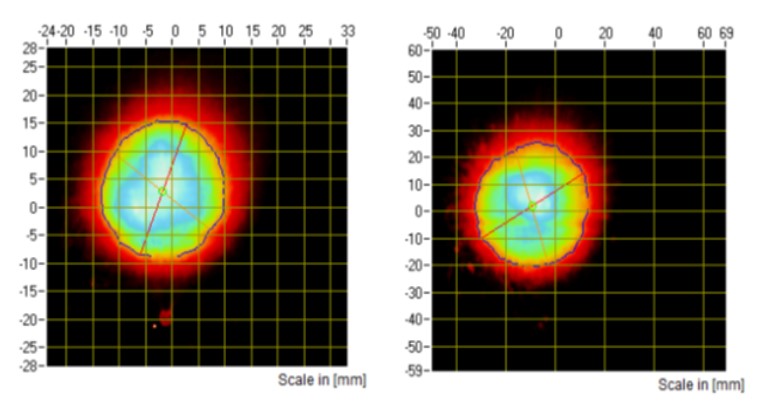
Figure 1: SP images from a nasal spray captured at 30 mm (A) and 60 mm (B) distances.
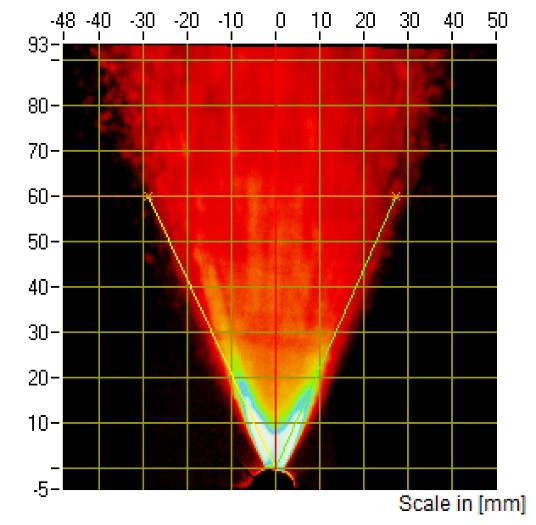
Figure 2: PG images from a nasal spray captured at 60 mm distance.
SP and PG testing are typically performed at the same tip-to-laser distance. For in vitro PBE studies of nasal spray drug products, SP will have to be measured at two distances, while PG testing will only have to be performed at one distance equal to the greater of the two distances selected for characterisation of the SP. The FDA recommends that these two distances be at least 3 cm apart within the range of 3–7 cm2. For these applications, SP measurement will either be performed at tip-to-laser distances of 3 and 6 cm or 4 and 7 cm (Figures 1 and 2).
For nasal spray applications other than PBE studies, SP determination at one distance is sufficient to meet regulatory requirements. However, collecting SP data at two distances is preferred in the case of MDIs, as the formation of aerosol plume may vary in shape (e.g. jet, mushroom), which will require plume characterisation at several distances in order to ensure full drug product understanding.
METHOD VALIDATION
“Working closely with CDMOs with dedicated experience in inhalation products can ensure that inhalation drug developers benefit from high precision testing capabilities to fully understand the performance of their chosen device and, in turn, gain a better idea of the therapeutic effect of their finished product.”
Validation of SP and PG methods should be completed before the start of the release and stability studies for the clinical/registration batches. SP and PG methods are typically validated for repeatability, intermediate precision and robustness as defined by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH).2
Repeatability expresses the precision under the same operating conditions over a short interval of time. Repeatability is typically assessed by one analyst performing replicate measurements on the same day using the same equipment.
Intermediate precision expresses variations within laboratories, such as different days, different analysts and different equipment. Intermediate precision is typically assessed by a second analyst performing the same number of replicate measurements on a different day using the same equipment.
The robustness of an analytical procedure is a measure of its capacity to remain unaffected by small but deliberate variations in method parameters and indicates its reliability during normal usage. Robustness for SP and PG methods may include variation of test parameters from the actuation portion of the method (e.g. velocity, stroke length) or from the laser portion of the method such as tip-to-laser distance, as well as spray duration (SP) or time delay (PG).
Acceptance criteria are typically set on Dmax, Dmin, ratio of Dmax/Dmin, and area for SP or plume angle and width for PG.
“SprayVIEW® measurement systems are valuable tools to guide drug product development. These systems can also support quality control for container closure components (e.g. valves, pumps) and finished drug products throughout the product’s lifecycle.”
BEYOND SPRAY PATTERN AND PLUME GEOMETRY TESTING
Spray characterisation studies should be initiated early on during the drug product development process to support device selection and formulation optimisation. Once the formulation has been finalised and the device selected, spray characterisation studies should be performed to assess product performance ahead of clinical studies. SprayVIEW® measurement systems are valuable tools to guide drug product development.
These systems can also support quality control for container closure components (e.g. valves, pumps) and finished drug products throughout the product’s lifecycle.
Additionally, specifications for OINDPs should include performance attributes of the pump such as minimum actuation force to achieve desired spray characteristics.3 Using the built-in actuation stations from the SprayVIEW® measurement system, actuation graphs can be collected as output parameters displaying the actuation profile, which, for instance, can be used to determine the force-to-actuate of the device (Figure 3).
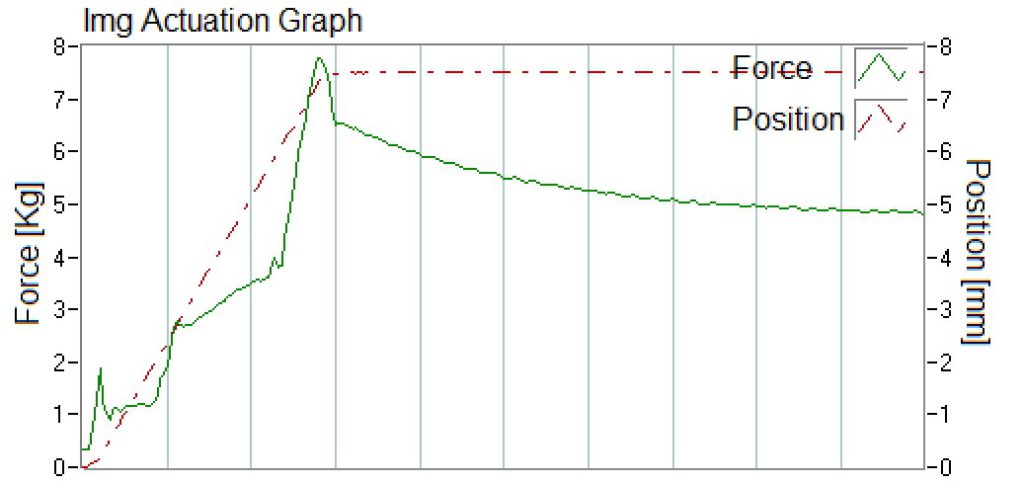
Figure 3: Actuation graph recorded during spray pattern measurement.
SprayVIEW® measurement systems can also be used to determinate the spray duration and spray velocity from an MDI. Time-synchronised image sequences of an aerosol plume are processed frame by frame starting when the plume first appears at the mouthpiece edge. The distance and associated time can be recorded for each image of the spray event until moving outside the camera’s field of view to allow for the determination of the emitted aerosol spray velocity (Figure 4). This methodology can be used to support comparison studies of spray duration and spray velocity between test samples.
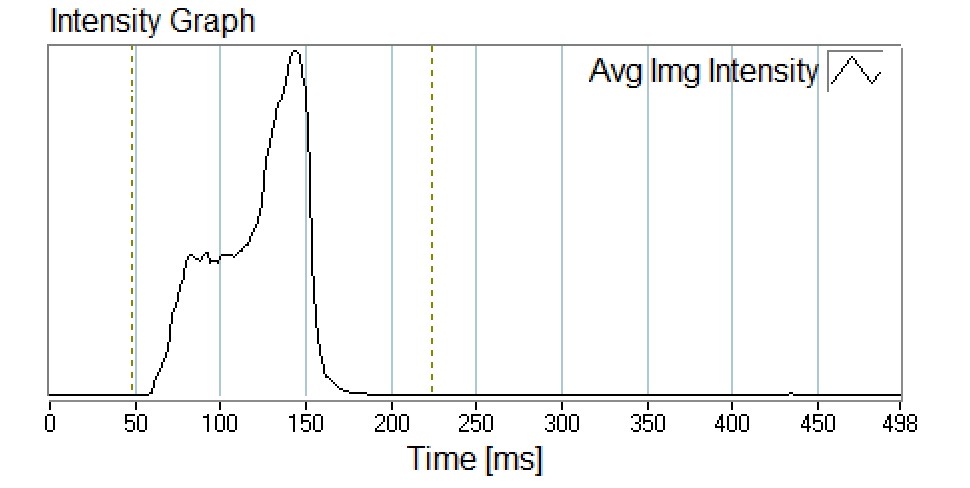
Figure 4: Intensity graph recorded during spray pattern measurement.
Other applications, including studies on the effect of ethanol concentration on pressurised MDI (pMDI) evaporation fraction using SprayVIEW® measurement systems, have been published by Proveris Scientific® in recent years. Finally, beyond the scope of OINDPs, SprayVIEW® measurement systems can support the spray characterisation for the development of nasal vaccines or of any other applications where the drug product is sprayed.
BENEFITING FROM SPECIALIST TESTING SUPPORT
In order to accurately gauge device performance, it is vital to have access to expert and specialist testing support. Working closely with CDMOs with dedicated experience in inhalation products can ensure that inhalation drug developers benefit from high precision testing capabilities to fully understand the performance of their chosen device and, in turn, gain a better idea of the therapeutic effect of their finished product.
Recipharm Inhalation Solutions™, for example, provides SP and PG testing services, supporting inhalation and nasal spray product development from initial formulation/process development to registration, product characterisation, commercial batch release and stability. The company performs in vitro PBE studies in both generic inhalation and nasal spray product development, and has expertise in developing and validating SprayVIEW®-based methods for aerosol and nasal spray testing. The company’s expert team also supports device formulation or process-related changes during product lifecycle management.
Working with a specialist inhalation CDMO offers considerable advantages for drug developers. Partnering with a company with an end-to-end service manages complexity and reduces risk. The company’s expertise in the segment means that it understands the unique challenges of inhalation drug development, and has the ingenuity to address them quickly, eliminating hurdles and minimising time to market.
By seeking the support of such partners, drug developers will be well placed to develop high-quality, truly effective inhalation products that will thrive in the competitive global pharmaceutical market and will transform patients’ lives for the better.
SprayVIEW® is a registered trademark of Proveris Scientific Corporation.
REFERENCES
- “Guidance for Industry: Bioavailability and Bioequivalence Studies for Nasal Aerosols and Nasal Sprays for Local Action”. US FDA, April 2003.
- “ICH Q2 (R1) Validation of analytical procedures: text and methodology”. European Medicines Agency, Published: November 1994 (part I); December 1996 (part II).
- “Guidance for Industry: Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products – Chemistry, Manufacturing, and Controls Documentation”. US FDA, July 2002.

