Citation: Sandler N, West T, “Nanoparticle Engineering and 3DP Delivery for Game-Changing Oral Therapeutics”. ONdrugDelivery, Issue 122 (Jul 2021), pp 9–13.
Niklas Sandler and Thomas West consider the technological advances in nanoparticle engineering that can improve the bioavailability and delivery of oral therapeutics.
“Innovations that can improve the bioavailability and solubility of small-molecule drug candidates could allow novel small-molecule therapies that would ordinarily have failed to achieve drug development success.”
A total of 85% of the most-sold drugs in the US and Europe are orally administered.1 With this in mind, technological advances that can improve the efficacy of orally administered therapeutics are vital. Nanoparticle engineering approaches can offer an effective means of improving the solubility, and thus bioavailability, of oral medications. This is significant because poor solubility leads to poor absorption in the gastrointestinal tract, meaning that drugs cannot reach their therapeutic target. For this reason, poor aqueous solubility is a major cause of failure for drug candidates in development.
While technologies such as nanoparticle engineering may be highly effective on their own for solving the challenge of poor solubility and bioavailability, collaboration within the industry is essential to fully optimise the power of new technologies. For example, 3D printing can facilitate therapies with rapid disintegration that are easier to swallow. By combining it with nanoparticle engineering, new opportunities can be created for enhanced patient-centric therapeutics (Figure 1). This article will look at the potential of nanoparticle engineering combined with industry collaboration to benefit patients worldwide.

Figure 1: Nanoform-branded tablet.
“Nanoform’s bio-nanoparticle technology is designed specifically for biologics, and can create stable biological nanoparticles as small as 50 nm. This is a break away from traditional methods that involve attaching the biologic to a drug delivery device, and holds the potential to significantly improve drug delivery of biologics by improving pharmacodynamic properties.”
ENHANCING THE PHARMACOKINETICS OF SMALL-MOLECULE DRUGS
Technologies that improve the pharmacokinetic properties of a drug candidate have the potential to set the stage for a new wave of game-changing oral small-molecule and biological drugs. The solubility of a compound is defined as its ability to dissolve in a solvent to produce a homogeneous solution.2 On the other hand, a drug’s bioavailability refers to the extent and rate at which the drug enters the systemic circulation in an unchanged form, thereby reaching its target area.3 These two variables are linked, with poor solubility being a factor that leads to poor bioavailability and makes it harder for new drug candidates to succeed. This is exacerbated by the present trend towards larger and more complex small-molecule drugs with greater molecular weights and hydrophobicity and, consequently, poor aqueous solubility.
The challenge this creates is evidenced by the difference between the solubility of drugs in development compared with those on the market. In total, 70–90% of in-development drugs fall into the low solubility categories of the Biopharmaceuticals Classification System (BCS).4 Meanwhile, fewer than 40% of drugs on the market fall under the same classification.1 Innovations that can improve the bioavailability and solubility of small-molecule drug candidates could allow novel small-molecule therapies that would ordinarily have failed to achieve drug development success.
OPENING UP NEW POSSIBILITIES FOR BIOLOGICS
Improving the pharmacokinetic properties of biologics also opens up new possibilities for drug delivery innovation in a rapidly growing market. Biologics, encompassing therapeutic proteins and other large biomolecules, as well as nucleic acid (DNA and RNA)-based therapies, have exploded in popularity in recent years. In 2020, seven of the top 10 best-selling drugs were biologics.5 The growth of the biologics market has been impressive and can be attributed in part to their high specificity, potency and safety profiles.
However, despite their success, bringing a biological drug to market comes with a number of challenges. Among these is that the cost of biologic production is high. Biologics can also have stability issues, which can cause loss of activity. Depending on several factors, including the administration route, biologics can also suffer from poor bioavailability. Improvements in technology that can allow the industry to access their potential are vital to help patients unlock the unique benefits of biologics. For example, enhancing the properties of biologics could pave the way for innovations such as an oral insulin formulation. This would remove the need for invasive injections for diabetics and could greatly improve their quality of life.
In light of this, nanoparticle engineering approaches that can improve bioavailability and drug delivery could lay the groundwork for more life-changing drugs to reach the patients who need them.
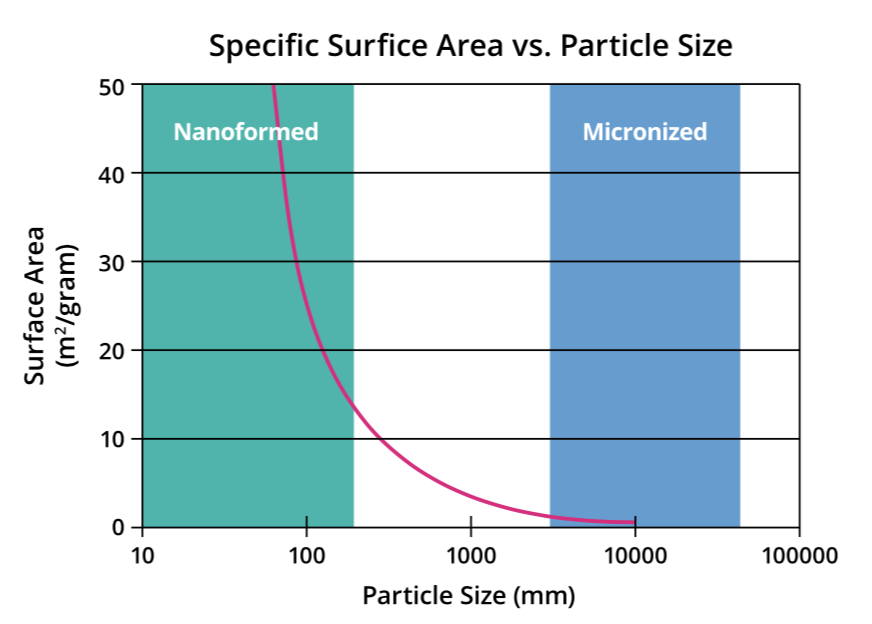
Figure 2: Graph illustrating the relationship between size and surface area for particles.
NANOPARTICLE ENGINEERING PRINCIPLES AND APPROACHES
Reducing the size of API particles increases their specific surface area. This leads to a proportional increase in dissolution rate that is especially noticeable at the nanometre scale, resulting in better absorption of poorly soluble drugs. For example, reducing particle size to 100 nm can result in a 30–40-fold increase in surface area from a 10 μm particle. Meanwhile, a particle of 50 nm can achieve a 1000-fold increase in specific surface area to a 10 μm particle. It is this principle that nanoparticle engineering approaches apply to improve the pharmacokinetic properties of drug candidates (Figure 2).
There are a number of nanoparticle engineering approaches on the market. For example, nanomilling is a technique that can successfully reduce drug particle size by milling in a wet medium. However, the use of mechanical energy to break up the nanocrystals raises surface free energy. This can result in amorphous domains on the crystalline material, introducing inherent instability which can make the API challenging to process further. The end product also requires excipients such as surfactants.
Spray-drying is an example of a popular micron-sized particle engineering method used for overcoming solubility issues in drug development. As the name implies, it works by spray-drying APIs with a polymer to create an amorphous solid and is effective for certain applications. Unfortunately, the technique can produce amorphous material that can create stability issues and make it more challenging to form tablets or capsules.
NANOFORMING DRUG PARTICLES WITH ENHANCED PERFORMANCE
Nanoform’s Controlled Expansion of Supercritical Solutions (CESS®) technology is a recrystallisation technique that dissolves and then crystallises small-molecule API particles from a solution of supercritical carbon dioxide (scCO2) without necessitating the use of excipients or changing the API’s inherent chemical properties. It is a controlled process, creating uniform particles that are tuneable in size, shape and polymorphic form. scCO2 is also a highly environmentally friendly solvent and offers excellent scalability. Using this technique, it is possible to create nanoparticles below 200 nm in size and, in some cases, as small as 10 nm. This is small enough to cross the blood-brain barrier, opening up exciting new possibilities for treating central nervous system (CNS) disorders, such as Alzheimer’s disease. In addition to creating opportunities for new therapies, this technology could also allow drugs that were previously discarded due to bioavailability issues to be revisited. In this way, CESS could double the number of drugs that reach clinical trials and, ultimately, the market.
Meanwhile, Nanoform’s bio-nanoparticle technology is designed specifically for biologics, and can create stable biological nanoparticles as small as 50 nm. This is a break away from traditional methods that involve attaching the biologic to a drug delivery device, and holds the potential to significantly improve drug delivery of biologics by improving their pharmacokinetic properties (Figure 3).
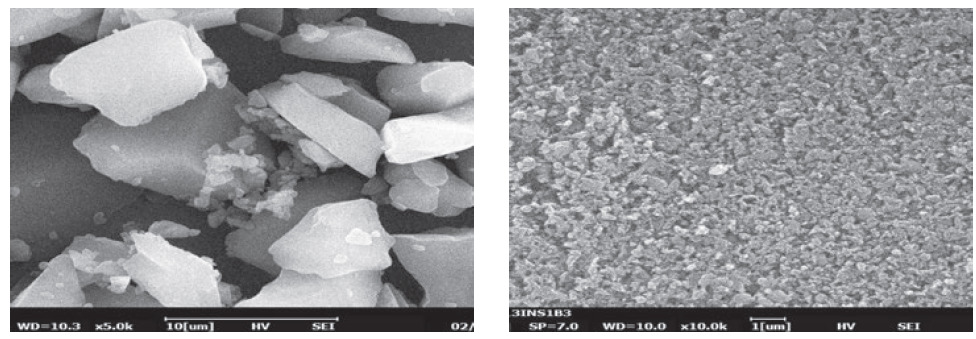
Figure 3: Microscopy images showing insulin particles before (left) and after (right) nanoforming.
“3DP is an entirely new way of making therapeutic dosage forms, and it can create novel structures that possess different functionality to those made using traditional manufacturing techniques. For example, Aprecia’s ZipDose® formulations enable dose loading of up to and beyond 1000 mg that disintegrate in the mouth in seconds when taken with a small sip of liquid.”
COLLABORATING TO PROVIDE ANSWERS
Collaboration in the industry is essential to bring new innovations to life. The capabilities of CESS have been highlighted by experimental results obtained through a collaboration with Pharmorphix® (acquired by Johnson Matthey) (Cambridge, UK) solid state services. The comparative in vitro dissolution study was carried out on piroxicam, an anti-inflammatory drug, and CESS-nanoformed piroxicam particles set a new benchmark in the industry. The release time for CESS-nanoformed particles was more rapid than those created using other industry-standard techniques, including spraydried amorphous dispersion, micron-sized, co-crystal, milled, and salt and hot-melt extrusion.
The rapid absorption of nanoformed piroxicam was demonstrated in a first-in-human trial of CESS-nanoformed piroxicam, carried out in collaboration with Quotient Sciences (Nottingham UK). The trial investigated the behaviour of single 20 mg nanoformed piroxicam immediate-release (IR) tablets in healthy volunteers, compared with 20 mg tablets of reference products, Feldene (Pfizer) and Brexidol (Chiesi).
Nanoformed tablets had a time of maximum plasma concentration Tmax of 1.75 h, earlier than both Felden and Brexidol (2.75 h and 2.25 h, respectively). The nanoformed piroxicam tablets exhibited an increased area under the curve (AUC) during the first hour after dosing. Achieved without the use of excipients, the faster absorption observed for nanoformed piroxicam compared with Brexidol provides strong evidence that nanoforming can enable simpler formulation strategies and higher drug loads in final products. This feature is of particular interest for therapies where rapid action is required, such as epilepsy.
Taken together, the results provide compelling evidence that nanoforming can facilitate simpler, faster-acting drug formulations with high drug loads. Further collaborations, however, continue to reveal new possibilities for nanoforming in the industry.

Figure 4: Tablets created using Aprecia’s ZipDose technology showing size progression.
A 3D-PRINTED NANOMEDICINE REVOLUTION
A partnership between Nanoform and Aprecia, a three-dimensional printing (3DP) pharmaceutical company, is set to explore how their respective technologies can be combined to create nanoparticle enabled
3DP dosage forms (Figure 4).3DP is an entirely new way of making therapeutic dosage forms, and it can create novel structures that possess different functionality to those made using traditional manufacturing techniques. For example, Aprecia’s ZipDose® formulations enable dose loading of up to and beyond 1000 mg that disintegrate in the mouth in seconds when taken with a small sip of liquid. So, for patients, it is swallowed like an oral solution or suspension. Moreover, ZipDose formulations are also efficient on a mass basis, often incorporating drug (or desired payload) as 40–60% of the total tablet weight (Figure 5).

Figure 5: ZipDose technology enables rapid disintegration in seconds.
The unique physical structure of ZipDose units is responsible for this differentiated capability. They are porous and uncompressed, created through selective binding of powder with liquid using the binder jetting style of 3DP. As a result, they resemble a powder that has been carefully “stitched together” using designed patterns of liquid droplets. This structure rapidly wicks liquid into the pores, which disconnects or dissolves the bridges between particles, leading to disintegration in the mouth within seconds. In this way, even very high dose medicines can be provided in an easy-to-swallow format using a small sip of liquid.
By combining ZipDose and CESS to create 3DP dosage forms containing nanoformed API particles, it may be possible to make high-dose medicines easier to take. CESS can improve the effective bioavailability of certain APIs, reducing the total dose of a drug required for the therapeutic effect. Meanwhile, ZipDose technology can be used to provide an easy-to-swallow formulation at any size, including reducing the pill count for medicines that require multiple tablets or capsules at each instance of dosing. The simple case above is of particular benefit to paediatric and geriatric patients, as these populations often have difficulty swallowing large, intact tablets and capsules. This phenomenon, known as dysphagia, affects over 16 million people in the US alone6 and could occur non-clinically in as many as 40% of Americans.
These improvements in dosage form efficiency, ease of administration and drug absorption could also enable combination therapies that would not have been possible before. For example, 3DP could facilitate a modular dosage form approach, building tablets up in sections with tailored release profiles, or APIs that are separated for stability reasons. The list goes on, with the potential applications of 3DP in combination with nanoparticle engineering promising to open up a new world of novel therapeutics for patients.
SMALL IS POWERFUL
Whether for small-molecule drugs or biologics, methods that can enhance oral drug delivery and bring novel therapies to market hold the potential to significantly improve patient outcomes. The latest nanoparticle engineering approaches offer a tidy solution to the poor solubility and bioavailability of new drug candidates, and could significantly improve patients’ quality of life through rapid-onset pills with potentially reduced adverse side effects. Collaboration within the industry is essential to fully realise the potential of new technologies. Combined with 3DP, new possibilities are created that can give hope to patients in all walks of life.
REFERENCES
- Behera BK, “Biopharmaceuticals: Challenges and Opportunities”. CRC Press, 2020.
- Savjani KT, Gajjar AK, Savjani JK, “Drug solubility: importance and enhancement techniques”. Int Scholarly Res Notices, 2012, Article ID 195727.
- Price G, Patel DA, “Drug Bioavailability”. StatPearls, Oct 2020.
- Kalepu S, Nekkanti V, “Insoluble drug delivery strategies: review of recent advances and business prospects”. Acta Pharm Sinica B, 2015 Vol 5(5), pp 442–453.
- Sagonowsky E, “The top 20 drugs by worldwide sales in 2020”. Fiercepharma.com. Accessed July 2021.
- Hamrang-Yousefi S, Goyal M, “Swallowing Study”. StatPearls, Mar 2021.

