To Issue 166
Citation: Fontanellaz T, Jost R, Schneider S, “Subcutaneous Drug Delivery: Adapting to High-Viscosity, Large-Volume Demands”. ONdrugDelivery, Issue 166 (Oct 2024), pp 8–12.
Thomas Fontanellaz, Reto Jost and Andreas Schneider discuss how Ypsomed is addressing the growing need for subcutaneous injections that accommodate higher volumes and viscosities, with innovative self-injection devices designed to enhance patient comfort and adherence.
The subcutaneous (SC) drug delivery landscape is undergoing significant change, fuelled by the increasing need for autoinjectors that are capable of handling high-viscosity, high-volume formulations. This shift is largely being driven by advances in biologics, which often require syringe-based autoinjectors that can accommodate larger volumes and higher viscosities.
“The recent introduction of handheld 2.25 mL prefilled syringe and autoinjector products has demonstrated the viability of delivering larger volumes, up to 2.0 mL.”
EXPANDING THE LIMITS OF INJECTION VOLUMES AND RATES
For years, the study of the upper feasible limits of injection volumes and rates has been a focus in the field of self-injection solutions. Traditionally, the industry has assumed that SC delivery was limited to administering up to 1.0 mL in 10–15 seconds. However, the recent introduction of handheld 2.25 mL prefilled syringe and autoinjector products has demonstrated the viability of delivering larger volumes, up to 2.0 mL,1 thereby expanding the possibilities for SC drug delivery.
Building on these advances, the simple and easy-to-use YpsoMate 2.25 has emerged as a key platform designed to address the growing need for higher-volume SC injections. With its intuitive two-step automatic injection process, YpsoMate 2.25 offers a reliable option for both clinical and commercial applications.
A key advantage of the YpsoMate 2.25 is its fill volume range of 0.4–2.25 mL, providing flexibility to accommodate different drug formulations. Its user-centric design simplifies the injection process, making it accessible to patients with limited dexterity or visual impairments, in line with the industry’s shift towards solutions that prioritise usability and adherence (Figure 1). In addition, the platform’s adaptability to different prefilled syringe, plunger and needle shield types supports a wide range of customer needs, from biotech firms to large pharmaceutical companies.
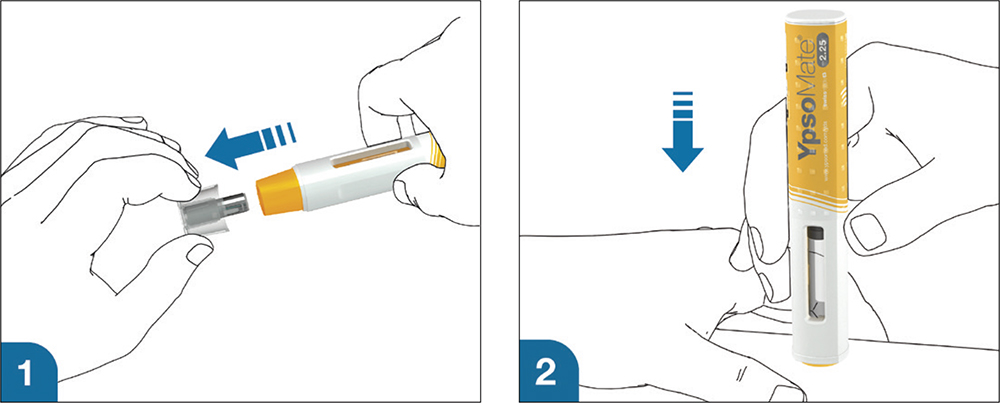
Figure 1: Handling steps of YpsoMate 2.25: a simple two-step mechanism – 1) Remove cap, 2) Inject.
The success of YpsoMate 2.25 lies in its technical capabilities and streamlined customisation process that de-risks development and reduces time to market. By centralising the critical stages of product development – such as design, human factors studies, clinical supply and regulatory filing – at its headquarters in Burgdorf, Switzerland, Ypsomed ensures that the platform can be precisely tailored to meet specific requirements while fostering seamless expert collaboration and efficient communication across all stages and maintaining high standards of safety and usability. This modular approach accelerates development timelines and reduces risk, enabling a rapid response to evolving market needs.
Integrating expert collaboration and risk management into the development process supports the delivery of innovative self-injection solutions globally.
INCREASING DOSE CAPACITY IN HANDHELD AUTOINJECTORS
As attention shifts to volumes greater than 2.25 mL, two emerging device categories are attracting significant attention. The first is the exploration of whether the established handheld autoinjector format can accommodate even larger volumes.2 Several investigational large-volume autoinjectors are now being developed with dose-volume capacities up to 10.0 mL. As previous research has shown that patients can safely and effectively hold devices on the skin for injections lasting up to 30 seconds, if not longer,1 industry efforts are increasing to expand the feasible upper volume limits for handheld devices.
Based on recent supportive literature and the emerging market need, Ypsomed introduced the YpsoMate 5.5, the newest member of the YpsoMate autoinjector family, which expands the design space of today’s handheld autoinjectors and enables the administration of injection volumes in the 2.0–5.5 mL range.
YpsoMate 5.5 is based on the proven YpsoMate 2.25 Pro technology and uses a similar type of constant force drive mechanism. This ensures that large drug volumes are reproducibly injected, even with high-viscosity formulations, which would not be possible with a conventional compression spring. Different spring configurations with different force profiles allow the system to be adapted to accommodate a wide range of drug viscosities, up to 30–50 cP. It also allows the flow rate to be adjusted to the desired target rate. Configurable injection times range from approximately 10 to 60 seconds for a 5.0 mL injection volume.
The YpsoMate 5.5 autoinjector also features the market-proven and widely accepted two-step handling principle: the user removes the cap and injects the drug by pushing the device against the skin. As with the other products in the YpsoMate family, the design is suitable for fully automated manufacturing. YpsoMate 5.5 is compatible with a wide range of applications and is designed as a cost-effective solution, facilitating efficient development and deployment (Table 1).
| Self-injection device | Max viscosity (cP) | Volume (mL) | Device type | User steps required | |
| Min | Max | ||||
| YpsoMate 2.25 | 25* | 0.4 | 2.25 | Handheld autoinjector | 2-step (remove cap, inject) |
| YpsoMate 2.25 Pro | 50* | ||||
| YpsoMate 2.25 with 8 mm needle | 40* | ||||
| YpsoMate 2.25 Pro with 8 mm needle | 80* | ||||
| YpsoMate 5.5 | 50* | 1.5 | 5.5 | ||
| YpsoDose | 50* | 2 | 10 | On-body patch injector | 2-step (patch, inject) |
*Feasibility testing is recommended for new drugs, as a broader range of viscosities can be accommodated.
Table 1: Overview of several Ypsomed self-injection devices, comparing viscosity handling, volume range, device type and user steps required for administration.
ON-BODY PATCH INJECTORS TO MEET LARGE-VOLUME DEMAND
Increases in dose volumes well beyond 5.0 mL remain tolerable in terms of pain and adverse events.2,3 Even when increases in dose volume are associated with increases in pain, these changes are often clinically insignificant and remain at the low end of absolute pain scales. For example, in a recent systematic review of the relationship between pain and injection volume, 15 of 16 studies reported only mild injection-related pain. While four studies found a significant positive relationship between injection volume and pain, five studies found no clinically relevant effect.2
The debate around the feasible upper limits of drug delivery volumes is likely to continue, particularly with respect to high-rate injections using handheld devices. At the same time, large-volume patch injectors have emerged as a new device category to facilitate the injection of even larger volumes.4 Whether user-filled, user-assembled, or prefilled and pre-assembled – with and without connectivity, and with volume capacities ranging from 3–50 mL – these devices share the goal of reducing injection rates for large-volume delivery. This approach aims to alleviate some of the challenges associated with large-volume, handheld devices. Several platforms are currently in clinical trials and some on-body injectors have been commercialised.5
The Ypsomed patch injector YpsoDose was developed in response to the growing demand for new large-volume SC therapies. These new therapies are usually antibody based and have payloads of up to 1,000 mg, if not more, requiring a fill volume of 5–10 mL. Ypsomed has harnessed its experience with prefilled pen injectors and autoinjectors to develop a modular and customisable patch injector platform to accelerate time to clinic, reduce up-front investments and lower project risks for pharma partners.
“YpsoDose addresses common challenges in the patch injector market, such as ensuring a consistent flow rate and minimising drug formulation loss.”
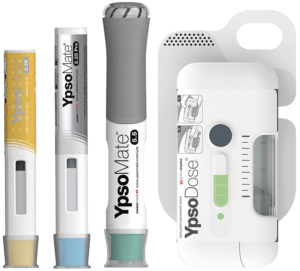
Figure 2: Ypsomed solutions for large volumes and high viscosities. Pictured from left to right: YpsoMate 2.25, YpsoMate 2.25 Pro, YpsoMate 5.5 and YpsoDose.
YpsoDose addresses common challenges in the patch injector market, such as ensuring a consistent flow rate and minimising drug formulation loss (Figure 2). Its prefilled system eliminates use steps that can lead to dosing variability, simplifying the process and improving safety by eliminating issues related to cartridge integrity and contamination. The fully assembled, ready-to-use design ensures consistent performance without complex final assembly steps, providing a streamlined, user-friendly experience. In addition, YpsoDose avoids the complexities associated with suction pump mechanisms, which can introduce limitations in pressure control and increase the number of parts in contact with the drug while injecting, potentially leading to contamination or residual volume issues.
The debate about user preferences for large-volume handheld devices versus on-body patch injectors is likely to continue. For example, Amgen’s recent decision to discontinue the PUSHTRONEX on-body device for Repatha® (evolocumab) is likely to intensify this discussion.6 Further research is needed to conclusively determine how injection durations and frequencies influence user preferences between the two emerging device categories for different patient populations – a complex issue that research is only beginning to explore.7
OVERCOMING THE CHALLENGES OF HIGH-VISCOSITY FORMULATIONS
The administration of high-dose biologics often requires formulations with higher solution concentrations, resulting in higher viscosities due to the well-characterised exponential relationship between antibody concentration and viscosity. This increase in solution viscosity presents several challenges, as it can negatively impact stability, manufacturability and drug delivery.3 While high-viscosity solutions have been associated with slightly increased injection site leakage, the volume leaked remains minimal, below 1% of the injection volume.9
Furthermore, research has uncovered surprising findings regarding injection pain – Berteau et al found that injections for high-viscosity formulations are less painful. Specifically, when comparing the perceived pain from injections of three different solution viscosities (1, 8–10 and 15–20 cP), the highest viscosity solutions were the least painful and most easily tolerated.10

Figure 3: YpsoMate 2.25 with 8 mm needle – optimised for high-viscosity formulations, enhancing patient comfort and injection efficiency.
The effect of viscosity on drug delivery has also been studied with respect to drug delivery devices and primary packaging.2,3 Higher viscosities require higher forces to expel the drug from the primary container, while ensuring that the primary container can withstand the increased force. This has led to innovations in primary packaging, such as the introduction of a new 27G ultra-thin-wall cannula with an external length of 8 mm, compared with the traditional 12.7 mm. Testing of this new design over a range of solution viscosities (2.3–30 cP) showed a significant reduction in injection time, particularly at higher viscosities.8 This has two important benefits – either autoinjectors with a pre-set force profile can now deliver higher viscosities at a constant injection time or the injection time can be reduced at a constant force, improving device usability and, ultimately, patient adherence to injectable treatments.
“The YpsoMate 2.25 has been optimised to further enhance patient comfort and ease of use.”
Recognising these challenges, the YpsoMate 2.25 has been optimised to further enhance patient comfort and ease of use. Notably, the introduction of an 8 mm external length cannula allows for greater versatility in accommodating high viscosity formulations (Figure 3). The extension of the proven platform for the new cannulas includes the design of new autoinjector parts to accommodate the shorter 8 mm needle, the manufacture and qualification of the corresponding injection moulds, the adaptation of the automated assembly process and extensive technical testing of the new YpsoMate 2.25 configuration. The shorter needle length, combined with a thinner wall, significantly reduces the force required to inject the medication. This improvement not only speeds up the injection process but also allows higher viscosities to be delivered in the same time frame. These advancements offer tangible benefits to both patients and healthcare providers, and the flexibility to choose between the 8 mm needle and the standard ½” needle offers pharmaceutical companies greater customisation options tailored to specific drug and patient needs.
Recent research suggests that both high-volume and high-rate injections are feasible and tolerated. This has further accelerated the demand for innovative self-injection systems targeting that segment. Ypsomed is at the forefront of the development of large-volume handheld autoinjectors and on-body patch injectors that offer flexibility in injection volume and solution viscosity to meet the diverse needs of pharmaceutical companies and patients (Table 1, Figure 4). These devices not only facilitate self-administration of complex medications, but also shift the point of care from the hospital to the home, increasing patient autonomy and reducing healthcare costs.
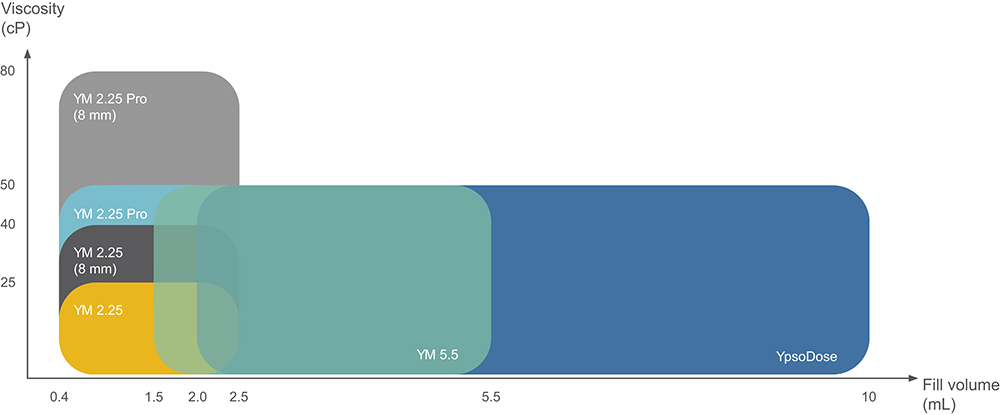
Figure 4: Viscosity (cP) versus volume (mL) for YpsoMate (YM) 2.25 autoinjectors, including models with the 8 mm needle configuration, YpsoMate 5.5 and YpsoDose.
REFERENCES
- Schneider A et al, “Hold the device against the skin: the impact of injection duration on user’s force for handheld autoinjectors.” Expert Opin Drug Deliv, 2020, Vol 17(2), pp 225–236.
- Schneider A et al, “Autoinjectors for large-volume subcutaneous drug delivery: a review of current research and future directions.” Expert Opin Drug Del, 2023, Vol 20(2), pp 1–16.
- Badkar AV et al, “Subcutaneous Delivery of High-Dose/ Volume Biologics: Current Status and Prospect for Future Advancements.” Drug Des Devel Ther, 2021, Vol 15, pp 159–170.
- Lange J et al, “Formative Study on the Wearability and Usability of a Large-Volume Patch Injector.” Med Devices (Auckl), 2021, Vol 14, pp 363–377.
- Woodley WD et al, “Clinical evaluation of large volume subcutaneous injection tissue effects, pain, and acceptability in healthy adults.” Clinical and Translational Science, 2022, Vol 15(1), pp 92–104.
- “One Repatha Presentation Will Be Discontinued Next Month, Another in 2025,” AIS Health, AIS Health Radar on Specialty Pharmacy, 2024, Vol 49(1).
- Schneider A et al, “Understanding patient preferences for handheld autoinjectors versus wearable large-volume injectors”. Expert Opin Drug Deliv, 2023, Vol 20(2), pp 271–283.
- Pager A et al, “User experience for manual injection of 2 mL viscous solutions is enhanced by a new prefillable syringe with a staked 8 mm ultra-thin wall needle”. Expert Opin Drug Del, 2020, Vol 17(10), pp 1485–1498.
- Doughty DV et al, “Understanding Subcutaneous Tissue Pressure for Engineering Injection Devices for Large-Volume Protein Delivery”. J Pharm Sci, 2016, Vol 105(7), pp 2105–2113.
- Berteau C et al, “Evaluation of the impact of viscosity, injection volume, and injection flow rate on subcutaneous injection tolerance.” Med Devices (Auckl), 2015, Vol 8, pp 473–484.

