To Issue 168
Citation: Shetty G, “510(k) Cleared, User-Filled Microdosing Device for Ophthalmic and Other Applications”. ONdrugDelivery, Issue 168 (Jan 2025), pp 120–123.
Gautam Shetty discusses unmet drug delivery needs in applications requiring accurate and precise microlitre-scale doses,going on to detail how the Congruence Medical’s recently cleared Microliter Dosing Syringe can bring the benefits of advanced syringe materials and performance to these applications.
Parenteral injections typically involve millilitre-scale volumes. However, since the approval and launch of Macugen® (pegaptanib, OSI Pharmaceuticals) in 2004 and Lucentis® (ranibizumab, Genentech) in 2006, more than 20 million1 microlitre intravitreal injections are estimated to take place every year, with more than 7 million2 injections in the US alone. Since then, other drugs targeting other diseases of the eye, such as geographic atrophy, and those involving microlitre injections have also been launched.
Outside of oncology, immunology and, more recently, obesity and metabolic disorders, ophthalmology is a key area of interest and investment for pharmaceutical companies. Beyond ophthalmology, applications such as local organ delivery for cell and gene therapy, intratumoural delivery and preclinical testing also involve injection of microlitre-scale doses.
AN UNMET NEED: USER-FILLED MICRODOSING DEVICE
Some approved ophthalmic drugs are currently available in a prefilled syringe format. While these meet the need for accuracy and precision, several drugs that are currently in clinical development or those drugs that cannot be prefilled even after approval, such as for cell therapies, have some or all of the following unmet needs.
Accuracy and Precision
Currently marketed syringes developed for the injection of millilitre volumes have been repurposed for injection of microliter volumes and are therefore inherently inaccurate and imprecise.3 These syringes have printed dose marks for reference; however, the accuracy of these markings does not impute delivery of an accurate dose volume – this is a common misunderstanding. Accuracy of dose delivery is limited by human capacity for axial manipulation of the plunger rod at the start and end of dose.
Overcome Dose-Marking Resolution
The resolution of a non-prefilled syringe with the lowest nominal volume approved for clinical use is 10 μL. Finer resolution is limited by the resolution of printing the dose marks by pad, screen or laser printing. Therefore, applications involving volumes that are not multiples of 10 μL cannot avail of off-the-shelf syringes.
High-Quality Drug Contact Materials
In certain sensitive applications, such as ophthalmology and neurology, conventional hypodermic syringes are unsuitable because of lubricants, unacceptable endotoxin content and/or other leachables. Some hypodermic syringe manufacturers have even issued a field safety notice asking users not to use their syringes for intravitreal injections due to the risk of leachable silicone. Additionally, hypodermic syringes may not conform to regulatory requirements for endotoxin content in sensitive applications. Furthermore, some silicone-free syringes have lubricants to replace silicone;4 therefore, not all silicone-free syringes are also lubricant-free. Also, the potential presence of perfluoroalkyl substances (PFASs) in contact with the drug should be avoided in light of REACH regulation in Europe and a recent lawsuit filed in the US by the state of Maryland involving PFASs.
“The benefits of high-quality syringe and plunger stopper materials developed for prefilled drugs are now available for drugs that need to be user-filled at the point of injection.”
Enabling Applications
with High Injection Forces Viscous formulations, fine injection needles, long delivery conduits or any combination thereof results in high injection forces. With currently available hypodermic syringes, there is a need to enable a comfortable injection. Two-piece syringes – syringes with no elastomeric plunger stopper – are known to leak past the plunger rod when attempting to inject viscous formulations.
Figure 1: Congruence’s MDS.
MICROLITER DOSING SYRINGE: A USER-FILLED MICRODOSING DEVICE
The US FDA recently cleared Congruence’s Microliter Dosing Syringe (MDS) that aims to address these unmet needs for non-prefilled drugs (Figure 1). This 510(k)-cleared device (K243149) is the first of its kind to incorporate a prefillable syringe for use with drugs that are filled in a vial and is indicated for intravitreal injections.
This means that the benefits of high-quality syringe and plunger stopper materials developed for prefilled drugs are now available for drugs that need to be user-filled at the point of injection (Table 1). This includes a silicone-free and lubricant-free syringe that conforms to USP <789> and whose extractables have been evaluated to be safe for intravitreal injections. The MDS meets stringent requirements to be classified as an ophthalmic syringe.
| Indication | Use in intravitreal injections |
| Intended Use | Inject fluid into or withdraw fluid from the body |
| Models | 9, 20, 25, 37.5, 50 and 100 μL models cleared |
| Dose Accuracy | ±3 μL for all models (95% confidence levels and reliability) |
| Usage | Single dose; disposable |
| Dosage Type | Fixed (one device configured to set and inject preset dose) |
| Needle Attachment | Luer lock (user-selectable needle) |
| Sub-visible Particulates | Conforms to USP <789>, USP <788> |
| Endotoxin | ≤ 0.2EU/mL |
| Biocompatibility | ISO 10993-1 compliant Tested for ocular irritation, intravitreal irritation |
| Sterilisation | Irradiation; provided sterile |
| Syringe Lubrication | None (silicone-free, lubricant-free) |
Table 1: MDS 510(k) Summary excerpt.
Each model of the MDS is configured to deliver a preset dose within 3 μL of the target volume. While few models, each with a different target injection volume, have been FDA cleared, additional models could be cleared using the current platform. Accuracy and precision of dose delivery for four different models are summarised in Figure 2. As shown, MDS models include target doses that are not possible with conventional user-filled hypodermic syringes – 9, 25 and 37.5 μL. Data from retina specialist users show accurate, precise microlitre injections when using the MDS technology.
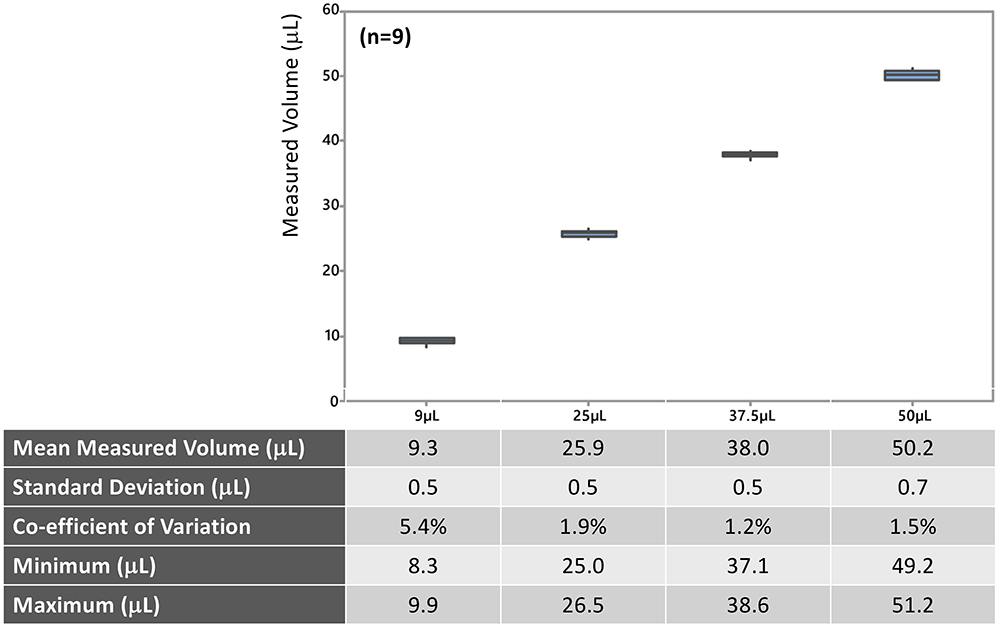
Figure 2 (n=9) Figure 2: Accuracy and precision of MDS (9, 25, 37.5 and 50 μL models).
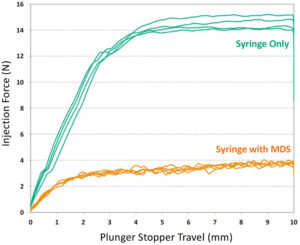
Figure 3: Attenuation of injection force.
Intravitreal injections involve fine-gauge needles – ideally 30G or finer to minimise pain and maximise patient comfort. Using a needle this fine increases the injection force, which is compounded by the increasing viscosity of drug formulations. Congruence’s MDS has been shown to inject formulations as viscous as 100 cP using a 30G ½” long needle. The MDS technology attenuates the injection force experienced by the user due to inherent mechanical advantage in its design and a further reduction in injection force experienced by the user from a reduction in flow rate, even when maintaining the same plunger rod speed. Figure 3 shows a comparison with and without the MDS when injecting a 50 cP formulation using a 30G ½” needle for the same plunger rod speed.
MAKING ANY SYRINGE A MICROLITER DOSING SYRINGE
The MDS technology is essentially a dose-metering plunger rod. This device architecture could make any syringe equivalent to a MDS, including prefillable syringes (Figure 4). The availability of a 510(k)-cleared embodiment could facilitate faster development and introduction of a prefilled device. Any such development can now leverage the market experience and usability data of the user-filled, 510(k)-cleared version.
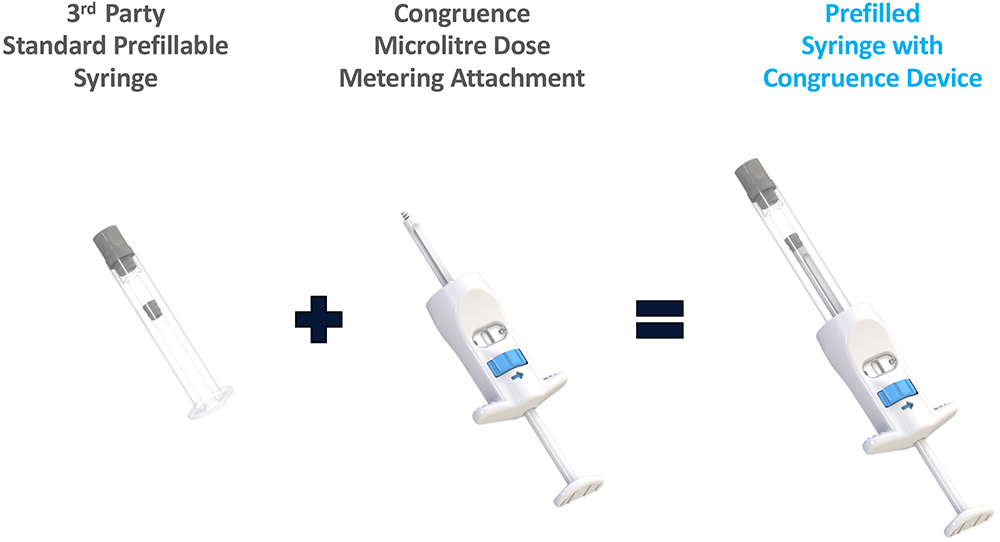
Figure 4: Prefilled embodiment – making any syringe a MDS.
CONCLUSION
Congruence’s MDS is a high-performing, high-quality, user-filled microdosing device that is now available for drugs, including for intravitreal injection, that are currently not prefilled in a syringe or those that cannot be prefilled in a syringe. The MDS has been shown to deliver accurate, precise injection volumes as low as 9 μL and has demonstrated the ability to inject viscous formulations with a fine-gauge needle. The MDS incorporates high-quality drug contact materials that were previously available only for prefilled drugs, opening them up to drugs that are (or have to be) user-filled, thereby also making it ideal for sensitive applications
REFERENCES
- Rimpelä AK et al, “Pharmacokinetic Simulations of Intravitreal Biologicals: Aspects of Drug Delivery to the Posterior and Anterior Segments”. Pharmaceutics, 2018, Vol 11(1), article 9.
- Wang R et al, “Quantifying burden of intravitreal injections: Questionnaire assessment of life impact of treatment by intravitreal injections (QUALITII)”. BMJ Open Ophthalmol, 2022, 7(1), article e001188.
- Agra LML et al, “Accuracy, Precision, and Residual Volume of Commonly Used Syringes for Intravitreal Injections and the Impact on Intraocular Pressure”. Ophthalmol Retina, 2023, Vol 7(10), pp 892–900.
- Dounce SM, Laskina O, Goldberg RA, “Particulate Matter from Syringes Used for Intravitreal Injections”. Retina, 2021, Vol 41(4), pp 827–833.

