To Issue 180
Citation: Farrow D, Mulpas J, Le Nuz S, “Orbital: A Novel High-Payload, Multi-Breath DPI Poised for Clinical Advancement.” ONdrugDelivery, Issue 180 (Nov 2025), pp 26–32.
David Farrow, Jonathan Mulpas and Solange Le Nuz discuss the patient-centric and data-driven development of Aptar Pharma’s Orbital dry powder inhaler platform, tracing its evolution from an initial concept to a clinically-ready device.
The therapeutic landscape for pulmonary drug delivery is increasingly defined by an urgent clinical need for high-dose therapies.1 Conditions such as cystic fibrosis (CF), non-CF bronchiectasis and severe pulmonary infections often require the administration of hundreds of milligrams of API directly to the lungs in order to achieve therapeutic efficacy.2 However, many conventional dry powder inhaler (DPI) technologies face fundamental limitations in this regard, often needing the device to be reloaded with multiple dose subunits to deliver the total required dose.
The particles required for deep lung deposition (typically 1–5 µm) are inherently cohesive due to their high surface-area-to-volume ratio and possess strong interparticle forces, leading to poor powder flow, agglomeration and inefficient aerosolisation. Consequently, existing devices often struggle to deliver large payloads consistently, which can lead to high oropharyngeal deposition and frequently require patients – many of whom have compromised lung function – to generate high inspiratory flow rates for effective drug aerosolisation.3,4
“THE STRATEGIC IMPETUS BEHIND THE ORBITAL PROGRAMME WAS TO DIRECTLY ADDRESS THE CHALLENGES OF THE HIGH-DOSE MARKET SEGMENT.”
In response to this significant unmet need, Aptar Pharma has developed Orbital a novel, high-payload, reloadable DPI platform (Figure 1). The strategic impetus behind the Orbital programme was to directly address the challenges of the high-dose market segment. Aptar has undertaken detailed engineering of Orbital’s aerosolisation engine, demonstrated its versatility with diverse and challenging formulations and assembled a robust preclinical data package that supports its advancement into clinical studies.
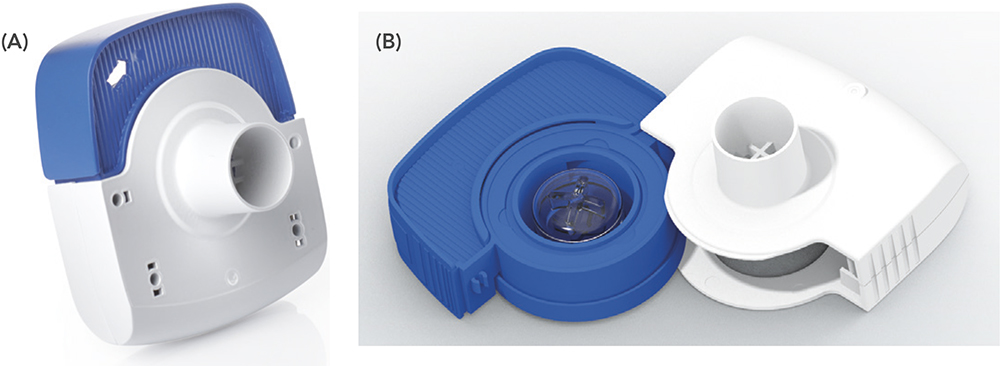
Figure 1: The Aptar Pharma Orbital device (a) in its closed state for storage or use and the (b) open configuration for loading.
THE ORBITAL ENGINE: ENGINEERING A POWERFUL AND DIFFERENTIAL DE-AGGLOMERATION MECHANISM
At the heart of the Orbital device platform is a unique and powerful aerosolisation engine designed to overcome the strong attractive interparticle energies of cohesive dry powders. The mechanism is fundamentally different from other DPIs, relying on a high-energy, multistage de-agglomeration processes, driven by the patient’s own inspiratory breath (Figure 2).
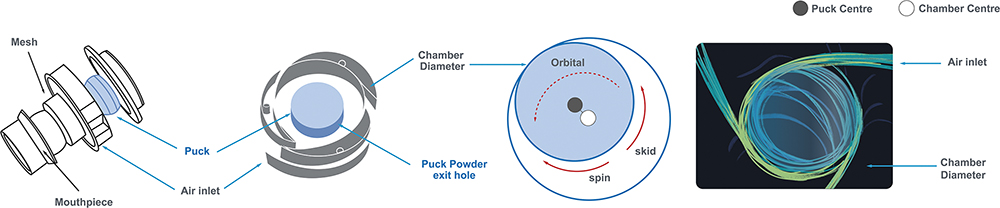
Figure 2: The core mechanism of the Orbital device.
The de-agglomeration process begins when the patient inhales. Air is drawn through strategically placed peripheral inlets in the chamber, creating tangential jets of air. This airflow propels the powder-containing reservoir, known as the “container”, into a high-speed orbital and elliptical motion around the chamber’s circumference. As the container orbits, its contact with the chamber wall induces a simultaneous spin on its own axis. This combined orbital and spinning movement creates a high-energy epicyclical motion, which is the primary driver of powder fluidisation and de-agglomeration. This dynamic motion facilitates a multistage de-agglomeration process that ensures the efficient dispersion of agglomerated powder into respirable primary particles:
- In-Container Fluidisation: The epicyclical motion imparts significant energy into the bulk powder within the container, initiating the break-up of loose agglomerates
- Controlled Emission: Centrifugal force progressively expels the fluidised powder through precisely engineered, rate-controlling orifices in the container wall
- In-Chamber Dispersion: The emitted particles enter the turbulent airflow of the de-agglomeration chamber, where further dispersion occurs through high-energy particle-particle and particle-wall collisions
- Final Sizing: The aerosolised powder cloud then passes through a grid in the mouthpiece, which serves as a final de-agglomeration stage before moving from the device into the patient.
“THE ORBITAL ENGINE IS SPECIFICALLY DESIGNED TO
IMPART SUFFICIENT AND SUSTAINED SHEAR AND IMPACT FORCES TO OVERCOME THESE COHESIVE BONDS, ENSURING HIGH EMITTED DOSE LEVELS AND AN FPF CAPABLE OF REACHING THE DEEP LUNG.”
This mechanism is engineered to address the fundamental physics of fine, cohesive powders. Particles in the respirable range are dominated by interparticle forces, such as van der Waals forces, that cause them to agglomerate and resist aerosolisation. The Orbital engine is specifically designed to impart sufficient and sustained shear and impact forces to overcome these cohesive bonds, ensuring high emitted dose levels and a fine particle fraction (FPF) capable of reaching the deep lung.
MULTI-BREATH DOSING WITH CONSISTENT PERFORMANCE – A KEY PATIENT BENEFIT
A critical innovation of the Orbital platform is its ability to deliver a large powder payload (e.g. 100–400 mg) over multiple, more gentle inspiratory breaths, unlike many traditional DPIs. By tuning the aperture size and number of the container’s exit orifices, the rate of drug emission can be precisely controlled. This allows the patient to inhale the full dose in several consecutive breaths rather than in a single, large inhalation, which often results in an uncomfortable bolus of powder.
Crucially, this design decouples the two primary functions of a DPI: dose emission and particle de-agglomeration. In many conventional devices, the energy from a single patient inhalation drives both processes simultaneously; a weaker breath results in both a lower emitted dose and poorer aerosolisation (i.e. a larger, less effective particle size).
The Orbital engine, however, separates these functions. The epicyclical motion, which requires only minimal airflow to initiate, is the primary de-agglomeration driver, ensuring that the quality of the aerosol remains high and consistent. The dose emission rate, meanwhile, is governed by the container’s orifices under centrifugal force. As a result, the aerodynamic particle size distribution remains remarkably consistent with each consecutive breath. This ensures that whether a patient with compromised lung function takes five gentle breaths or three stronger ones, the particles delivered to the lung are of a consistently optimal size for therapeutic efficacy, representing a significant advance in device reliability and patient-centric design.
A PATIENT-CENTRIC DEVELOPMENT PATHWAY FROM CONCEPT TO CLINICAL READINESS
“RECOGNISING A BROADER MARKET OPPORTUNITY, THE APTAR PHARMA TEAM INITIATED A PROGRAMME TO TRANSFORM THE DEVICE FROM A DISPOSABLE, SINGLE-PRODUCT FORMAT INTO A VERSATILE, RELOADABLE PLATFORM CAPABLE OF ADDRESSING
THE HIGH-DOSE MARKET.”
The journey of the Orbital device from a nascent concept to a clinically-ready platform was guided by a rigorous, iterative and patient-centric development philosophy. This process began with the strategic acquisition of the intellectual property for a single-use mannitol device in 2022. Recognising a broader market opportunity, the Aptar Pharma team initiated a programme to transform the device from a disposable, single-product format into a versatile, reloadable platform capable of addressing the high-dose market.
Human factors engineering was integrated at the earliest stages of development through Aptar Pharma’s Noble subsidiary. Initial concepts and early prototypes were evaluated to gather direct feedback on usability and ergonomics. This research was critical in identifying key areas for improvement, such as a flimsy drawer mechanism that participants felt lacked a proper seal, confusing container loading orientation and suboptimal ergonomics that made the device difficult to grip securely. This direct user feedback became the catalyst for targeted engineering enhancements. The development team initiated an iterative design process, leading to more advanced prototypes that systematically addressed these usability challenges. The initial drawer was replaced with a robust integrated sliding mechanism, and the device’s external housing was reshaped to improve grip and handling.
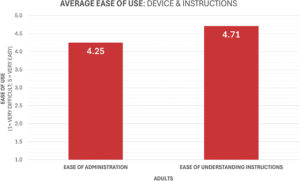
Figure 3: Findings from the human factors study.
Subsequent usability validation confirmed the success of this patient-centric approach. The refined Orbital device was found to be intuitive, with participants reporting a high level of confidence and rating the device as “easy to use” (Figure 3). This research also provided more nuanced feedback that informed final design considerations. For instance, observations highlighted the potential need for a dose-completion feedback mechanism to help users to track the multi-breath dosing sequence. User feedback also indicated a five-to-three preference for a digital inhalation counter over a visualisation window, providing a clear directive for future smart device integration, which is currently well underway at Aptar. This continuous loop of design, testing and refinement has ensured that the final Orbital device is not only technically proficient but also demonstrably usable and tailored to the needs of its intended patient populations.
UNLOCKING NEW THERAPEUTIC POSSIBILITIES – FORMULATION VERSATILITY FOR SMALL MOLECULES AND BIOLOGICS
A defining feature of the Orbital platform is its ability to effectively aerosolise a wide spectrum of powder formulations, from conventional blended powders to highly challenging engineered particles, which are often required for biologic modalities. The device’s robust performance has been extensively demonstrated with standard carrier-based formulations, which typically consist of a micronised API blended with a larger excipient carrier, such as lactose monohydrate. The development programme has included comprehensive testing of itraconazole (ITZ) formulations blended with both lactose and mannitol, confirming the platform’s capability to efficiently de-agglomerate and deliver these common powder classes.
The delivery of biologic modalities, such as proteins, peptides and nucleic acids, via the pulmonary route represents one of the most significant challenges in drug delivery today.5 These molecules are notoriously “fragile” and highly susceptible to the physical and chemical stresses encountered during formulation and aerosolisation. Shear stress, heat and moisture exposure can lead to denaturation and aggregation, resulting in a loss of therapeutic activity and the potential for immunogenic responses.6
To overcome these stability issues, biologics are typically formulated into inhalable particles using advanced engineering techniques such as spray-drying or freeze-drying, often with stabilising excipients, such as polyols or polymeric additives. While these processes create stable particles, the resulting powders are often amorphous, porous and highly cohesive, making them extremely difficult to disperse using conventional, low-energy DPIs.5
“THE ORBITAL PLATFORM IS UNIQUELY POSITIONED TO SOLVE THIS CRITICAL “FORMULATION-DEVICE PARADIGM”. ITS HIGH-ENERGY DE-AGGLOMERATION ENGINE PROVIDES THE FORCE NECESSARY TO OVERCOME THE STRONG COHESIVE BONDS OF THESE ENGINEERED PARTICLES, ENSURING THAT THEY ARE DISPERSED INTO PRIMARY PARTICLES SUITABLE FOR DEEP LUNG DEPOSITION.”
The Orbital platform is uniquely positioned to solve this critical formulation-device paradigm. Its high-energy de-agglomeration engine provides the force necessary to overcome the strong cohesive bonds of these engineered particles, ensuring that they are dispersed into primary particles suitable for deep lung deposition. At the same time, the brief residence time within the engine and the nature of the dispersion forces minimise exposure to destructive shear stresses that could damage the fragile biologic modalities. Furthermore, the formulation is protected from ambient moisture ingress with a sealed container, sealed in a blister or pouch (with desiccant if required) until the moment of use – a key advantage for hygroscopic amorphous biologic powders.
This capability transforms Orbital from a simple delivery device into an enabling platform technology that can significantly de-risk the development of inhaled biologics. These biological modalities are often extremely expensive compared with traditional small-molecule therapeutics, so achieving efficient, consistent dosing while ensuring long shelf lives is more important than ever for developers and payers. By providing a pre-validated, high-performance delivery solution for such challenging powders, the Orbital platform enables developers to focus on optimising their formulation with the confidence that a compatible and effective delivery system already exists. This has the potential to unlock a new generation of inhaled biologic therapies that were previously considered undeliverable.
FUTURE-PROOFING INHALATION THERAPY – DESIGNING FOR DIGITAL INTEGRATION
The future of respiratory medicine is increasingly intertwined with digital health technologies. “Smart inhalers” are emerging as powerful tools to address two of the most persistent problems in inhalation therapy: poor patient adherence and incorrect inhaler technique.4 Published data indicate that up to 90% of patients make at least one critical error in their inhaler technique, which can lead to suboptimal disease management and an increased burden on healthcare systems.7,8
From its inception, the Orbital platform has been designed with this digital future in mind. The findings from the human factors studies provided clear clinical justification for this approach; the observed use error where participants failed to complete the required number of inhalations is precisely the type of issue that can be mitigated by a connected device. The strong user preference for a digital counter further validates this development trajectory.
The device’s design readily accommodates the integration of sensors and communication modules. A “Smart Orbital” device has already been developed in collaboration with the Aptar Digital Health group and an initial proof-of-concept version is already active in testing. Future versions are likely to incorporate a range of functionalities with the potential to enhance patient outcomes and provide valuable data for clinicians:
- Adherence and Dose Completion: On-board sensors could track each inhalation, providing patients with real-time feedback and reminders to ensure that the full multi-breath dose is administered correctly
- Technique Monitoring: Integrated airflow sensors could measure inspiratory flow rate and duration, offering patients feedback to optimise their inhalation technique for optimal drug delivery
- Data for Clinicians: Data on adherence patterns and inhalation technique could be transmitted via Bluetooth to a secure application and clinician portal, providing objective insights to guide personalised treatment adjustments.
The reloadable nature of the Orbital device, where the core hardware is reused, aligns perfectly with the environmental sustainability and long-term use model of a connected health device, requiring only the replacement of the disposable container. This forward-thinking design ensures that the platform is not only ready for today’s clinical challenges but is also prepared for the next wave of innovation in digital respiratory care.
CLINICAL APPLICATION – A CASE STUDY IN INHALED ITZ FOR PULMONARY ASPERGILLOSIS
To validate the Orbital platform, Aptar Pharma has undertaken a comprehensive development programme using ITZ as a model compound for treating pulmonary aspergillosis. This programme serves as a powerful case study, demonstrating the device’s capabilities with a challenging molecule in an area of significant unmet medical need.
The management of chronic and allergic pulmonary aspergillosis presents a significant clinical challenge, necessitating therapies that can achieve high local drug concentrations within the airways while minimising systemic toxicity. ITZ, a broad-spectrum triazole, is a therapeutic cornerstone for pulmonary aspergillosis, but its use in oral formulations is frequently complicated by a narrow therapeutic window, variable bioavailability, extensive drug-drug interactions and dose-limiting toxicities, including hepatotoxicity and cardiotoxicity.9,10 Consequently, the development of an inhaled formulation to deliver ITZ directly to the site of infection has been a long-standing goal in respiratory medicine.
The primary objective for an inhaled antifungal is to deliver a sufficient mass of drug to the lungs to exceed the minimum inhibitory concentration (MIC) for target pathogens, such as Aspergillus fumigatus, which typically ranges from 0.125 to 2.0 µg/mL.11–13 Achieving this requires overcoming the substantial formulation challenges posed by ITZ’s physicochemical properties, namely its high cohesiveness and poor aerosolisation efficiency.
Developmental efforts for DPI formulations of challenging compounds such as ITZ have historically yielded modest fine particle doses (FPDs) – the critical measure representing the mass of drug particles likely to reach the therapeutic target within the lungs. Early-stage clinical investigations confirmed the feasibility of the inhaled route but were often constrained by the payload limitations of existing DPI technologies.
While specific, peer-reviewed FPD data for ITZ using this technology is limited, its delivery efficiency potential can be illustrated using the TOBI Podhaler® (tobramycin – Viatris, Canonsburg, PA, US), a marketed product using Pulmosphere™ (Novartis, Basel, Switzerland) particles. Clinical scintigraphy studies in healthy volunteers demonstrated that TOBI Podhaler® achieved a mean whole-lung deposition of 34% of the nominal dose, significantly higher than nebulised tobramycin,14 with an FPD per capsule of ~9.5 mg. Although tobramycin has different physicochemical properties than ITZ, this demonstrates the high efficiency achievable with this engineered particle approach.
Techniques such as advanced spray drying are employed to create particles with desirable aerodynamic properties, often targeting high FPFs. Research on inhaled voriconazole, another azole antifungal with similar delivery challenges, formulated via spray drying with leucine as an excipient, reported achieving FPFs up to approximately 60.8% using specific devices.15 Extrapolating this FPF to a hypothetical high-performance ITZ formulation using a similar technique highlights the FPD constraints. For example, delivering a 10 mg nominal dose with 90% device emission and 60% FPF would yield an FPD of roughly 5.4 mg, which is still within the low-to-mid single-digit milligram range.
These examples, while using advanced formulation techniques, underscore a persistent limitation in delivering a sufficiently high FPD (i.e. tens of milligrams) of drugs such as ITZ in a single, convenient administration. This often necessitates multiple daily actuations to achieve a cumulative therapeutic dose, which can compromise patient adherence and limit overall efficacy.
The Orbital platform represents a paradigm shift in this context. By using both high-concentration blends and particle-engineered formulations, alongside the high-efficiency, high-payload device, Aptar has developed a DPI system capable of delivering an FPD of ITZ exceeding 20 mg in a single dose. This was determined from a formulation exhibiting an emitted fraction of ~74% and an FPF of the emitted dose of ~74%, from a 37% w/w formulation containing ITZ, loaded with a formulation mass of 100 mg into a container. This result constitutes a 5- to 10-fold increase in the deliverable FPD compared with previously reported systems in clinical development (Figure 4).
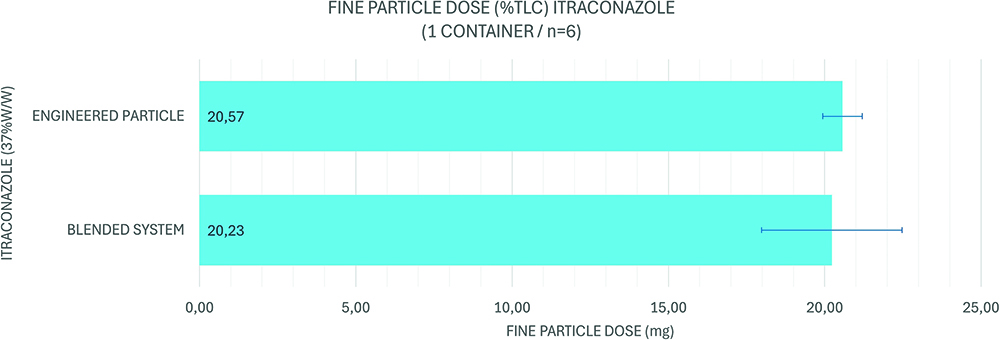
Figure 4: Current state of ITZ formulations for Orbital.
The therapeutic implications of achieving such a high lung dose are profound. A single dose delivering over 20 mg of ITZ is predicted to generate local concentrations in the airway mucosal lining that are orders of magnitude above the MIC90 for A. fumigatus. This has the potential to enable more effective treatment regimens, improve fungal clearance, overcome emerging antifungal resistance and reduce the treatment burden for patients. Furthermore, by maximising local delivery, this high-payload system maintains the critical safety advantage of the inhaled route, minimising systemic exposure, thereby circumventing the toxicities associated with high-dose oral ITZ therapy. The ability to deliver such a high dose with a gentler inspiratory breath is also highly advantageous, as those suffering with the disease may find deep breaths more challenging.
CONCLUSION
This exhaustive development work – from its strategic inception to address the high-dose market, through patient-centric design evolution, to its validation with a challenging clinical candidate – demonstrates its maturity and readiness. Aptar Pharma’s Orbital is now poised to enter clinical studies, offering a compelling and powerful new solution for partners seeking to overcome the most significant challenges in pulmonary drug delivery.
REFERENCES
- Tarara TD et al, “Formulation of Dry Powders for Inhalation Comprising High Doses of a Poorly Soluble Hydrophobic Drug”. Front Drug Deliv, 2022, Vol 2, art 862336.
- Labiris NR, Holbrook AM, Chrystyn H, “Dry Powder versus Intravenous and Nebulized Gentamicin in Cystic Fibrosis and Bronchiectasis. A Pilot Study”. Am J Respir Crit Care Med, 1999, Vol 160(5), pp 1711–1716.
- Ke W-R et al, “Administration of Dry Powders During Respiratory Supports”. Ann Transl Med, 2021, Vol 9(6), art 596.
- Jansen EM et al, “Global Burden of Medication Non-Adherence in Chronic Obstructive Pulmonary Disease (COPD) and Asthma: A Narrative Review of the Clinical and Economic Case for Smart Inhalers”. J Thorac Dis, 2021, Vol 13(6), pp3846–3864.
- Lo JCK, Pan HW, Lam JKW, “Inhalable Protein Powder Prepared by Spray-Freeze-Drying Using Hydroxypropyl-β-Cyclodextrin as Excipient”. Pharmaceutics, 2021, Vol 13(5), p615.
- Rostamnezhad M et al, “Spray Freeze-Drying for Inhalation Application: Process and Formulation Variables”. Pharm Dev Technol, 2022, Vol 27(3), pp 251–267.
- “Evidence Reviews for Smart Inhalers”. NICE Guideline No. 245. National Institute for Health and Care Excellence, 2024.
- Zabczyk C, Blakey JD, “The Effect of Connected ‘Smart’ Inhalers on Medication Adherence”. Front Med Technol, 2021, Vol 3, art 657321.
- Yang W, Wiederhold NP, Williams RO III, “Drug Delivery Strategies for Improved Azole Antifungal Action”. Expert Opin Drug Deliv, 2008, Vol 5(11), pp 1199–1216.
- Halpern AB et al, “Primary Antifungal Prophylaxis During Curative-Intent Therapy for Acute Myeloid Leukemia”. Blood, 2015, Vol 126(26), pp 2790–2797.
- Pfaller MA et al, “Wild-Type MIC Distribution and Epidemiological Cutoff Values for Aspergillus fumigatus and Three Triazoles as Determined by the Clinical and Laboratory Standards Institute Broth Microdilution Methods”. J Clin Microbiol, 2009, Vol 47(10), pp 3142–3146.
- Burgel P-R et al, “High Prevalence of Azole-Resistant Aspergillus fumigatus in Adults with Cystic Fibrosis Exposed to Itraconazole”. Antimicrob Agents Chemother, 2012, Vol 56(2), pp 869–874.
- Tashiro M et al, “Correlation Between Triazole Treatment History and Susceptibility in Clinically Isolated Aspergillus fumigatus”. Antimicrob Agents Chemother, 2012, Vol 56 (9), pp 4870–4875.
- Newhouse MT et al, “Inhalation of a Dry Powder Tobramycin PulmoSphere Formulation in Healthy Volunteers”. Chest, 2003, Vol 124(1), pp 360–366.
- Arora S et al, “Highly Resipirable Dry Powder Inhalable Formulation of Voriconazole With Enhanced Pulmonary Bioavailability”. Expert Opin Drug Deliv, 2016, Vol 13(2), pp 183–193.

