To Issue 180
Citation: Harley P, Al-Amari M, Jones M, “How Cutting Edge in Silico Approaches Can Reduce Time and Risk in Development of Inhalation Devices”. ONdrugDelivery, Issue 180 (Nov 2025), pp 62–67.
Dr Peter Harley, Mariam Al-Amari and Matt Jones explore how in silico approaches can transform pulmonary and nasal drug delivery device development, illustrating through case studies – spanning low-global-warming potential propellants, assembly robustness, device-user interaction, variability and capsule piercing dynamics – how simulation-led in silico approaches can improve understanding of device performance and identify risks early, reducing development costs and accelerating the path to more reliable, innovative and patient-friendly drug-device systems.
Pulmonary and nasal drug delivery are among the most complex routes of administration to engineer for successfully, as device performance is highly influenced by the nuances of both the drug formulation and user behaviour. Pressurised metered dose inhalers (pMDIs), dry powder inhalers (DPIs), soft mist inhalers (SMIs) and nasal delivery devices must operate reliably under a wide range of use scenarios, manufacturing variabilities and environmental conditions. Typically, development programmes rely heavily on limited sets of highly controlled physical tests on prototype devices that represent a small range of possible manufacturing variation. While these tests are necessary, they frequently fail to capture the complete variability of real-world use. This creates a significant gap in knowledge and understanding that can lead to expensive late-stage or on-market manufacturing and performance issues.
Conventional development programmes are usually constrained by the risks that engineers can anticipate. If there is limited understanding of the way that drug-device combinations will behave with real-world use, the breadth of risks that can be anticipated and mitigated with good design or manufacturing controls will also be limited.
Tolerance analyses and physical tests tend to be the workhorses of most design and development programmes. In practice, however, it is the unanticipated and poorly understood conditions – or combinations of conditions – such as component misalignments or deflections at tolerance extremes, storage outside expected ranges or non-ideal user technique, that most often drive late-stage device failure. These difficult-to-quantify extreme conditions and hidden variables can compromise device robustness, manufacturing reliability and therapeutic efficacy.
“BY APPLYING COMPUTATIONAL MODELLING AND SIMULATION METHODS DURING DEVICE DEVELOPMENT, ENGINEERS CAN EXPLORE AND UNDERSTAND VAST ARRAYS OF VIRTUAL SCENARIOS AND CONFIGURATIONS FEATURING VARYING GEOMETRY, MATERIALS, ENVIRONMENTS AND USER INTERACTIONS, ENABLING THEM TO PREDICT DEVICE PERFORMANCE ACROSS THE ENTIRE DESIGN SPACE.”
The use of in silico approaches to design and evaluate inhalation devices provides a deeper understanding of the modes and limits of performance. This understanding generates opportunities to create more successful designs, minimise development timelines and provide enhanced insights that can unlock next-generation engineering concepts. By applying computational modelling and simulation methods during device development, engineers can explore and understand vast arrays of virtual scenarios and configurations featuring varying geometry, materials, environments and user interactions, enabling them to predict device performance across the entire design space. In silico approaches can now be adopted from pre-design and development activities all the way through to supporting regulatory submissions, as outlined by the American Society of Mechanical Engineers (ASME) V&V40 standard.
“AS COMPUTING POWER AND THE SOPHISTICATION OF SOFTWARE INCREASES, IN SILICO APPROACHES ARE BEGINNING TO UNLOCK PREVIOUSLY UNKNOWN INSIGHTS INTO DEVICE PERFORMANCE, AS WELL AS THE ROBUSTNESS OF THE DEVICE-USER INTERFACE.”
COMPUTATIONAL MODELLING AND SIMULATION IN THE DESIGN OF INNOVATIVE PULMONARY AND NASAL DRUG DELIVERY DEVICES
Next-generation development approaches are being enabled by rapidly evolving technologies. Device engineers can deploy in silico approaches to deepen their understanding of why a device behaves as it does and identify unknown and unexpected risks so that they can be mitigated before a device reaches critical development phases, dramatically reducing the risk of a development programme’s failure. As computing power and the sophistication of software increases, in silico approaches are beginning to unlock previously unknown insights into device performance, as well as the robustness of the device-user interface. These approaches can be applied to a broad range of device types, including pMDIs, DPIs, SMIs and nasal sprays with delivery intended either to the lung (via the nose and mouth) or to the nasal cavity (and potentially onward to the brain).
Modern computational modelling and simulation (CM&S) techniques can cover a wide range of performance areas, as summarised in Table 1. Design and performance in these areas can be optimised to ensure that inhalation devices are robust, patient-friendly and have predictable performance in real-world conditions.
| Performance Area Examples | What Simulation Can Help Designers Understand |
| Thermal effects | Impact of temperature on device performance |
| Material variability | Impact of material strength variation on device performance |
| Capsule or blister evacuation | Efficiency and completeness of drug release |
| Deagglomeration | Powder break up |
| Drug deposition (upper airway versus deep lung) | Distribution profile and therapeutic targeting |
| Aerodynamic particle size distribution (APSD) | Particle size exiting the device and its effect on deposition |
| Resistance tuning | Optimisation for user inhalation profiles |
| Capsule robustness | Sensitivity to humidity, bevel geometry and robustness |
| Mouthpiece/nosepiece geometry | Seal with lips or nose, ergonomics and user comfort |
| Robustness under external effects | Gravity, blocked inlets, tolerance extremes, device orientation and freefall drop |
| Nasal delivery positioning | Effect of position in the nose on deposition |
| Breath profile robustness | Impact of pressure drop and user-to-user variability |
Table 1: Performance areas addressable by in silico approaches.
USING IN-SILICO APPROACHES TO DE-RISK DEVICE DEVELOPMENT
Identifying Risks Associated with Low-GWP Propellants in pMDIs
With existing propellants such as HFC-134a and HFC-227ea accounting for a significant proportion of the carbon footprint of the pharmaceutical industry, the drive to move to low carbon alternatives is strong. However, the switch to low global warming potential (GWP) propellants such as HFO-1234ze and HFA-152a has introduced new challenges for device developers – challenges that can be de-risked and understood using in silico approaches.
One relevant unknown may be related to a system’s resuspension dynamics as the device is shaken. Understanding these dynamics through simulations can enable instructions for use, suitability assessments and predictions of dosing profiles and spray plume dynamics. These and similar risks can be better understood either before the first devices are built or following physical tests on a limited number of devices. The fidelity and accuracy of the simulation’s predictions can be enhanced by using real-world data from users, captured using sensor-enabled devices, as inputs.
Decoding an Assembly Issue
Simulating components in their nominal geometry and positioning is incredibly valuable for understanding the detailed modes and mechanisms by which a device functions. However, certain failure modes only become apparent when components are considered at the extremes of their design tolerance. In silico approaches can be coupled with a design of experiments (DoE) approach to decode challenges such as failed assembly steps.
In such studies, virtual device populations are generated, simulated and examined to unveil multifactor drivers on key assembly steps across the full range of relevant tolerances (Figure 1). This can involve developing simulations to assess in excess of 1,000 virtual assemblies. Importantly, creating physical prototypes that represent even a very small number of these tolerance combinations can be challenging, time consuming and expensive. By using a simulation-DoE approach, statistically significant variables can be extracted, enabling existing tolerance definitions to be challenged, part costs to be reduced and the assembly process to be improved to maximise yields with minimised downtime.
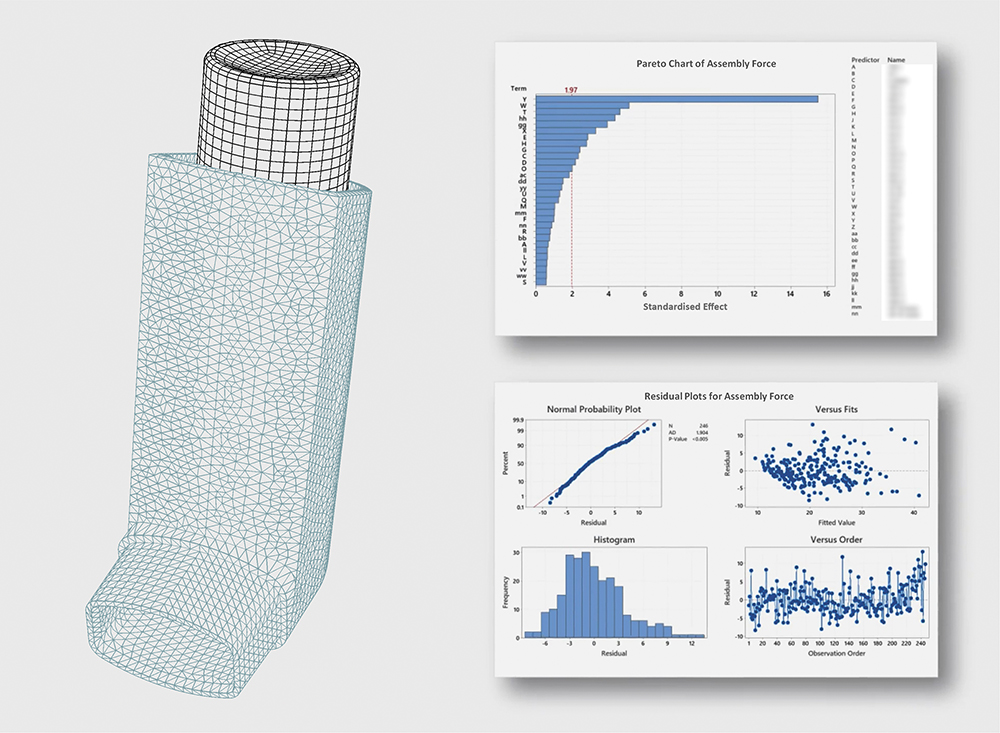
Figure 1: Assembly steps can be simulated to understand the effect of component tolerance variation, allowing prediction of the impact of new tooling being introduced, for example.

Figure 2: pMDI actuation simulation into an anatomically representative human mouth and throat demonstrating suspension impaction and propellant evaporation.
Understanding Device-User Robustness
It is widely reported that pMDIs suffer from significant deposition in the mouth and throat of a patient. When a patient uses any type of inhaler, the angle at which they hold the device in their mouth or nose will have a noteworthy impact on the final drug lung deposition. Without recruiting a single participant, in silico approaches can be used to determine the sensitivity of the device to non-ideal user actuation, such as a patient accidentally pointing the nozzle towards their tongue; simulating this exact scenario has shown that lung deposition could swing from 6–8% of the metered dose, to <1% for a worst-case mouth/throat impaction (Figure 2).
Deep lung localised deposition can be modelled using next-generation numerical lung modelling, further enabling the ability to estimate the best- and worst-case deposition profiles for a range of patients, which in turn can inform predictions of drug efficacy variability that may be seen during clinical trials. Then, flipping the requirement to targeted deposition, the same in silico techniques can be deployed to understand the robustness of nasal dosing to targeted zones to support the next generation of devices capitalising on the nose-to-brain delivery pathway.
Exploring DPI Capsule Piercing Risks
Capsule piercing success is typically experimentally assessed via powder evacuation measurements. However, the mechanism of piercing and subsequent design variables of interest are difficult to understand experimentally. CM&S can be used to determine ideal piercing geometry, as well as to uncover the risk of powder migration immediately post puncture. CM&S can also enable rapid exploration of system variables on overall system performance, with example variables being capsule material strength, piercing geometry and component tolerances (Figure 3). Again, these techniques can be used both to optimise design before tooling investments, to provisionally explore device performance, and to understand why a prototype is not performing as expected after production.
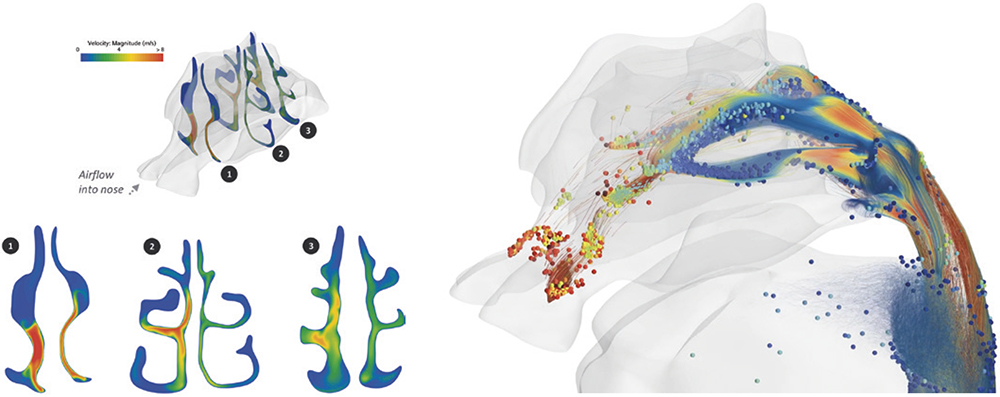
Figure 3: DPI inhalation simulation into an anatomically representative human nasal passage demonstrating flow paths and powder deposition patterns.
IN SILICO PLUS ADVANCED PHYSICAL TESTING FOR REGULATORY SUBMISSIONS
The ASME V&V40 standard provides a framework to support the credibility assessment of models generated using in silico approaches, with an aim of integrating the modelled outputs as regulatory evidence. Interpretation of V&V40 for any given scenario requires subject matter experts to review the context of use (COU) with respect to the Question of Interest. Establishing credibility goals and designing a relevant V&V40 plan requires an in-depth understanding of not only the simulated physics, but also the risk associated with the COU. Modelling experts routinely develop a range of simulations that replicate standard testing, an example being the robustness of the actuation counter to ensure target functionality is achieved, as described in ISO 20072.

Figure 4: Anatomically representative human nasal passage airflow ducting used to quantify deposition patterns in experimental characterisation campaigns.
Alongside pushing boundaries in simulation, it is important to step beyond the standard physical test methods to develop devices that are reliable and robust in the full variety of real-world manufacturing and use scenarios. For example, the next-generation impactor (NGI) test was designed and introduced in the late 1990s and adopted widely in the early 2000s – now, more than 20 years later, it is often as far as developers go in understanding the delivery performance of their drug-device system. By embracing the latest testing technologies and method developments, it is possible to further enhance analysis and understanding of device performance and robustness, while also providing further credibility for in silico simulations (Figure 4).
As an example, laser diffraction testing is regularly used in specialist test laboratories to characterise aerosol performance (Figure 5). Coupling this with in silico approaches can enable a rapid assessment of a device’s ability to aerosolise its formulation and provide a direct link to the predicted anatomical deposition patterns. Furthermore, anatomically representative geometries can be introduced into the test method such that the characteristics of the aerosol after impaction in the mouth and throat can be captured and used for higher fidelity deep-lung simulations. This capability to front-load development programmes with sophisticated de-risking activities ensures that when the time comes for final design verification (DV) and clinical testing, the performance is well understood and the risk of encountering costly issues is dramatically reduced.

Figure 5: Anatomically representative human mouth and throat airflow ducting used to quantify deposition patterns in laser diffraction testing.
Once an in silico model’s credibility has been established, virtual populations unlock the ability to complement DV testing with qualified virtual results. Depending on the COU and risks associated with the model-based decision making, these simulated virtual populations can enable test quantities to be reduced, saving significant effort in the production of real-world devices.
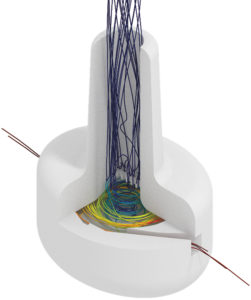
Figure 6: Example of a DPI airflow simulation used to tune device resistance and understand powder transport.
CONCLUSION
Embracing the latest technologies across in silico techniques alongside advanced experimental methods can provide an avenue to revolutionise existing device development methodologies, with the ultimate objectives of:
- Developing a deeper understanding of how drug-device-patient systems behave and why, unlocking enhanced insights to enable better designs
- More rapidly optimising designs and minimising prototyping tooling loops, resulting in reduced time to market
- Reducing late-stage and on-market challenges caused by a lack of insight and understanding, de-risking the development programme.
The examples and case studies summarised here indicate the range of opportunities available in the pulmonary and nasal delivery device space, where physics-based modelling and simulation and advanced physical testing can not only help identify risks and potential mitigations but also deepen understanding of drug-device-patient systems to unlock novel thinking for the next generation of device technologies (Figure 6). Introducing in silico approaches can also automate evidence generation, reducing costs and accelerating development.

