To Issue 181
Citation: Arora B, “Drug Delivery in Cell and Gene Therapy: Why Early Device Development Matters”. ONdrugDelivery, Issue 181 (Dec 2025), pp 48–51.
Bharat Arora discusses how the delivery method for a cell and gene therapy is an integral part of the overall product, inextricable from the therapy itself, and how properly synchronising drug and delivery device development can greatly benefit the overall development programme.
A NEW ERA, NEW DELIVERY CHALLENGES
Cell and gene therapies (CGTs) are reshaping modern medicine; from chimeric antigen receptor T-cell (CAR-T) therapies to in vivo CRISPR treatments, these products offer a curative potential once considered impossible. However, alongside their promise lies a less glamorous, yet absolutely critical, reality – no matter how advanced the science, the therapy is only as effective as its delivery system.
“IN CGTs, THE DELIVERY SYSTEM IS OFTEN MORE THAN A CONTAINER OR CONDUIT – IT IS A DETERMINANT OF EFFICACY, SAFETY AND USABILITY.”
In CGTs, the delivery system is often more than a container or conduit – it is a determinant of efficacy, safety and usability. Be it the infusion system that keeps CAR-T viable, the intrathecal catheter that precisely delivers an adeno-associated virus (AAV) vector or the prefilled syringe that ensures accurate dosing, each of these devices is inseparable from the therapy’s clinical outcome.
However, in many development programmes, delivery systems are treated as an afterthought. The drug or cell product drives early-stage attention, while device integration is deferred until the later clinical phases. This imbalance creates major risks, including delays in clinical trials, increased regulatory scrutiny, the need for redesigns and even post-approval issues.
THE ASYMMETRY OF DRUG AND DEVICE DEVELOPMENT
One reason delivery systems lag is that drugs and devices live in different development universes:
- Drugs and biologics follow a well-trodden path of preclinical toxicology, clinical phases and chemistry, manufacturing and controls (CMC)
- Devices are governed by engineering principles, design controls and iterative usability testing.
A level of integration between drug development and device development teams is crucial during early-stage development. When teams are integrated correctly, drug development efforts provide desirable inputs to design control elements for the device (Figure 1).
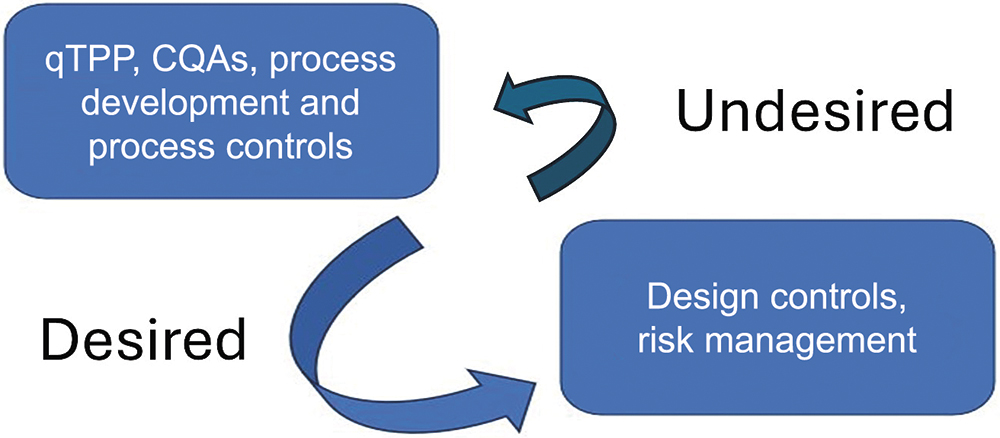
Figure 1: Desired and undesired information flow between drug and device development. (qTPP: Quality Target Product Profile, CQA: Critical Quality Attribute)
“DRUG AND DEVICE TIMELINES RARELY ALIGN; A GENE THERAPY VECTOR MAY BE READY FOR PHASE I TRIALS LONG BEFORE THE CATHETER OR INFUSION SET IS FULLY CHARACTERISED. CONVERSELY, A PROVEN DEVICE MAY NOT MEET THE UNIQUE NEEDS OF FRAGILE BIOLOGICS.”
However, drug and device timelines rarely align; a gene therapy vector may be ready for Phase I trials long before the catheter or infusion set is fully characterised. Conversely, a proven device may not meet the unique needs of fragile biologics. For small molecules, late device integration can sometimes be tolerated, but, for CGTs, the delivery interface defines whether or not the product works at all.
Table 1 shows a high-level side-by-side comparison of drug and device development activities during early development. The US FDA’s guidance “Current Good Manufacturing Practice Requirements for Combination Products” provides guidance on how cGMP requirements apply to combination products, and how device and drug regulations overlap.
| Drug Development | Device Development |
| Formulation Development (Target Product Profile) | Design Inputs (User Needs, Device Requirements, Use/Hazard Analysis) |
| Process Development (Critical Quality Attributes, Critical Process Parameters, Stability) | Design Outputs (Specifications, Prototypes, Essential Requirements) |
| Process Qualification (Engineering Runs, Characterisation Studies) | Design Verification and Validation |
| Process Risk Assessment | Risk Management File (Risk Plan, Design/Use/Process Risk Analysis, Risk Report) |
Table 1: Side by side comparison of early-stage drug and device development activities.
WHY EARLY DEVICE DEVELOPMENT IS NON-NEGOTIABLE
A more pragmatic, risk-based approach is to treat drug and device as co-equal from day one. The FDA guidance “Early Development Considerations for Innovative Combination Products” provides valuable insights on scientific, technical and regulatory issues when combining drugs, biologics and devices early in development. Delaying device development can trigger cascading problems:
- Regulatory Delays: Agencies expect evidence of drug-device compatibility before pivotal trials. The FDA’s Center for Biologics Evaluation and Research (CBER) and Center for Devices and Radiological Health (CDRH), as well as the EMA’s Committee for Advanced Therapies (CAT), all emphasise early alignment
- Clinical Risk: Suboptimal devices can compromise cell viability, affect biodistribution or alter pharmacokinetics
- Manufacturing Disruption: Device-related variability (e.g. shear forces, adsorption, extractables and leachables) can invalidate batches
- User Failure: Poorly designed systems can increase dosing errors or reduce adherence, especially in outpatient or home settings
- Cost Escalation: Redesigns late in development often require bridging studies or new stability data, adding millions to programme costs.
When the investigational product is a gene therapy, the FDA’s guidance “CMC Information for Human Gene Therapy IND Applications” offers recommendations on how to include device or delivery aspects.
FIT-FOR-PURPOSE DEVICES: ENGINEERING FOR BIOLOGY
During early development stages, developers should characterise the device not as a commodity but as an enabler of the therapy. Critical considerations include:
- Physical Forces: Shear stress in tubing or pumps can rupture cells or destabilise viral capsids
- Volume and Viscosity: Many gene therapy formulations are highly viscous, requiring specialised syringes or autoinjectors
- Container Closure Integrity: AAV vectors or messenger RNA (mRNA) are sensitive to oxygen and moisture, so their primary packaging must protect their integrity
- Delivery Accuracy: Variability in residual volume can alter the delivered dose by clinically meaningful margins
- Temperature Control: Cryopreserved cell therapies demand devices that are compatible with cold chain workflows.
The concept of essential performance requirements or essential drug delivery outputs, borrowed from device regulations, should be generally applied to biologics. For example, in a prefilled syringe, break-loose and glide forces are not just ergonomic metrics; they determine whether or not a fragile biologic is delivered intact. The article “Opportunities and Challenges in Biologic Drug Delivery” provides useful information on delivery challenges (e.g. viscosity, stability, adsorption) for biologics that parallel those for CGTs.1
LESSONS FROM REAL-WORLD EXAMPLES
The following examples highlight that the challenges of delivery do not have a universal solution – it must be engineered to suit the specific biology of each therapy. The article “Watershed Year of Cell and Gene Therapy (CGT): A Review” provides additional examples on the evolving landscape and momentum of CGTs to supplement those presented here.2
CAR-T Therapies
Approved CAR-T therapies, such as Kymriah (tisagenlecleucel, Novartis) and Yescarta (axicabtagene ciloleucel, Kite Pharma, Los Angeles, CA, US), rely on infusion bags and transfer sets designed to preserve cell viability. Early development challenges included cell loss due to adsorption on tubing surfaces and viability drops linked to pump systems. These issues underscored the importance of testing device-cell interactions before scaling manufacturing.3
Spinal Delivery of AAV Gene Therapies
Intrathecal delivery of AAV vectors, as in Zolgensma (onasemnogene abeparvovec-xioi, Novartis) clinical programmes, required specialised catheters to ensure accurate placement and flow rates. Regulatory agencies scrutinised these delivery systems closely, recognising that off-target dosing could create severe risks.
Prefilled Syringes for mRNA Vaccines
While not CGTs, mRNA vaccines highlighted the importance of syringe compatibility, where adsorption of lipid nanoparticles to syringe surfaces was a critical risk, necessitating material-specific testing. This lesson can be directly applied to future in vivo gene therapies.
GLOBAL REGULATORY COMPLEXITY
Combination products straddle regulatory silos. For example, in the US:
- CBER/CDER regulate the biologic component
- CDRH governs device performance
- Final jurisdiction depends on the “primary mode of action”.
In Europe, the EMA’s CAT evaluates advanced therapy medicinal products (ATMPs), with device conformity assessed under the Medical Device Regulation (MDR). Japan, China and other regions apply their own hybrids of drug and device frameworks.
The 21st Century Cures Act in the US and the MDR in the EU both elevate the importance of early drug-device integration. Global developers must plan for divergent but overlapping requirements. The article “A Regulatory Risk-Based Approach to ATMP/CGT” discusses regulatory strategies for both the EU and the US, as well as risk-based planning for advanced therapies.4
“ENGAGING REGULATORS EARLY ISN’T OPTIONAL – IT IS A SURVIVAL STRATEGY FOR CGT DEVELOPERS.”
KEY TESTING PRIORITIES FOR CGT COMBINATION PRODUCTS
Engaging regulators early is not optional – it is a survival strategy for CGT developers. Some key priorities include:
- Extractables and Leachables: Detecting impurities that migrate from device materials into biologics
- Drug-Device Interactions: Adsorption of proteins, viral vectors or cells onto device surfaces
- Stability Under Delivery Conditions: Ensuring that biologics remain intact during infusion, injection and storage
- Performance Testing: Residual volume, dose accuracy, injection forces and flow rates
- Usability and Human Factors: Validating device operation under real-world conditions.
LIFECYCLE PLANNING: BEYOND FIRST APPROVAL
Delivery challenges do not end with approval. Lifecycle changes – new manufacturing sites, material substitutions and production scale-up – can all impact product quality. Regulators increasingly require “line of sight” planning that anticipates post-approval changes.
Smart developers use a total product lifecycle model, embedding feedback loops from clinical trials, supply chains and post-market surveillance back into design. By implementing transparency across suppliers and CDMOs, drug developers can ensure continuity throughout product development and commercialisation.
FUTURE HORIZONS: DIGITIAL, CONNECTED AND SUSTAINABLE DELIVERY
The CGT market is continually growing. While these therapies are currently focused on managing monogenic disorders produced by a mutation in a single gene, such as severe combined immunodeficiency, muscular dystrophy and haemophilia, the scope of CGTs is increasing to target more complicated multigenic diseases, such as tumours, cardiac disease and diabetes, by changing the expression of multiple genes instantaneously.
According to Nova One Advisor (Pune, India), the global CGT market size is expected to be worth around US$119.30 billion (£90.78 billion) by 2034, increasing from $25.89 billion in 2025, representing a healthy compound annual growth rate of 18.5% from 2025 to 2034.5 And the future of CGT delivery will not stop at syringes and catheters – emerging trends include:
- Connected devices that track dosing, flow rates and patient adherence
- Digital twins for device-drug systems, enabling predictive modelling of performance
- Sustainable materials, driven by a push from regulators and patients for greener supply chains
- Platform devices, where a single delivery system supports multiple therapies, reducing redundancy.
For CGTs, these innovations could be game-changers – but only if planned early.
“TREATING DRUG AND DEVICE AS CO-EQUAL FROM THE OUTSET REDUCES RISK, ACCELERATES APPROVALS AND ENSURES THAT PATIENTS RECEIVE THERAPIES THAT ARE BOTH SAFE AND EFFECTIVE.”
CONCLUSION: DELIVERY DEFINES SUCCESS
The message is simple – in CGT, the delivery system is not a separate component; it is the therapy’s partner. Treating drug and device as co-equal from the outset reduces risk, accelerates approvals and ensures that patients receive therapies that are both safe and effective. By merging quality by design with design controls, embracing risk-based integration and effectively planning the development lifecycle, developers can transform delivery from a bottleneck into a differentiator. In the race to bring curative therapies to patients, those who master delivery will define the future of CGTs.
Disclaimer: The statements expressed here are those of the author or an external reference source and do not reflect the opinion or position of Vertex Pharmaceuticals.
REFERENCES
- Hooven MD, “Opportunities and Challenges in Biologic Drug Delivery”. American Pharmaceutical Review, Dec 2017.
- Jian A et al, “Watershed year of cell and gene therapy (CGT): A review of 2024 CGT approvals”. Cancer Lett, 2025, Vol 632, art 217980.
- Awasthi R et al, “Kymriah® (tisagenlecleucel) – An overview of the clinical development journey of the first approved CAR-T therapy”. Hum Vaccin Immunother, 2023, Vol 19, art 2210046.
- Salazar-Fontana LI, “A Regulatory Risk-Based Approach to ATMP/CGT Development: Integrating Scientific Challenges With Current Regulatory Expectations”. Front Med (Lausanne), 2022, Vol 9, art 855100.
- “Cell and Gene Therapy Market Size Expected to Hit USD 119.30 Billion by 2034”. Press Release, BioSpace, Sep 2025.

