To Issue 181
Citation: Gill S, Suttie A, “Precision Delivery: Seamless Collaboration for Delivering Neuro-Therapeutics”. ONdrugDelivery, Issue 181 (Dec 2025), pp 52–55.
Professor Steven Gill and Alan Suttie provide insight into the unique collaboration and design philosophy behind their novel scalable interventional delivery system for gene and cell therapies – the Neurochase Acute Drug Delivery System.
A NEW PARADIGM FOR INTRAPARENCHYMAL DRUG DELIVERY
In the rapidly evolving landscape of gene and cell therapies, the challenge of safely and effectively delivering treatments directly to the brain in a scalable manner remains a critical hurdle. For therapies targeting neurological conditions, systemic administration is often ineffective – and potentially toxic – with less than 1% of the dose reaching the target.
Whilst existing intraparenchymal delivery methods are effective, they involve lengthy and complex surgeries that will make them challenging to roll out for more common indications. Instead, meeting this need will demand a precision-guided approach that needs to be faster and more effective than the currently available options. Together, Neurochase and Fearsome, have developed the Neurochase Acute Drug Delivery System (Figure 1) – an innovative approach that emphasises a tight synergy between deep clinical expertise and engineering to overcome fundamental physiological and logistical challenges.
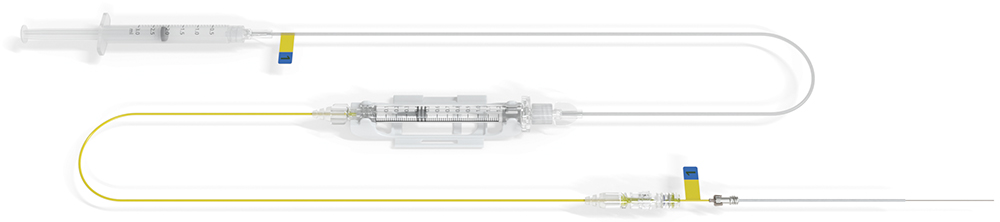
Figure 1: The Neurochase acute drug delivery system.
The foundation of the project’s success arises from a single point of inspiration. Neurochase’s unique combination of decades of surgical leadership in intraparenchymal drug delivery together with an intuitive understanding of physiology and engineering, provided the Fearsome team with an unparalleled starting point. This vision was not just about the device, but rather the critical interface between the synthetic device, therapeutic agent and delicate brain physiology.
PRIORITISING THE END RESULT: CONTROLLED INFUSION
The Neurochase Acute Drug Delivery System was developed from a singular focal point – the ultimate delivery of the therapeutic. The key objective of the system is that it has to produce a certain shape of infused volume within the brain at the end of the procedure. Achieving this required mastering an extraordinarily complex technical problem: controlled volume infusion. As an analogy, imagine trying to blow up a balloon without there actually being a skin on the balloon. The team needed to model fluid behaviour influenced by precise flow rates, cannula tip pressure and the unique features designed into the system.
“IF THE THERAPY IS ADMINISTERED SYSTEMICALLY, ONLY ABOUT 0.1% OF THE DOSE GIVEN ENTERS THE BRAIN – THE REST OF IT GOES TO THE LIVER AND IS QUITE TOXIC. AS SUCH, IT IS IMPORTANT TO HAVE TIGHT CONTROL OF THE DOSE AND MAKE SURE THAT IT ENDS UP IN THE RIGHT PLACE.”
The necessity of direct delivery is driven by the biology of gene therapy. For most gene therapies, it is critical that sufficient numbers of viral particles penetrate each cell to ensure that the therapy actually works. If the therapy is administered systemically, only about 0.1% of the given dose enters the brain – the rest of it goes to the liver and is quite toxic. As such, it is important to have tight control of the dose and make sure that it ends up in the right place.
TACKLING TECHNICAL HURDLES: REFLUX CONTROL
One of the most persistent technical challenges in intraparenchymal drug delivery is managing reflux – the backwards flow of infusate along the cannula track, which can lead to insufficient dosing at the target and potentially toxic dose accumulation outside the target area. The development team’s solution diverges from traditional “point source” delivery, where a very fine tube going into the brain tissue creates a tissue seal. This produces a spherical volume, but creates a source of very high pressure at a single point.
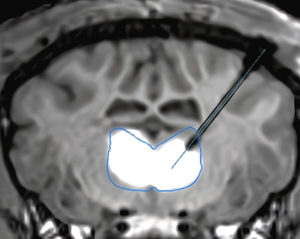
Figure 2: Magnetic resonance imaging (MRI) showing a real-time infusion into the thalamus using the Neurochase Acute Drug Delivery System of a gene therapy co-labelled with MRI contrast agent gadolinium to enhance visibility.
To overcome this, the final design of the Neurochase Acute Drug Delivery System focuses on distributing the infusate along the exposed length of the cannula to maintain lower pressure at any single point, minimising tissue damage and maximising distribution (Figure 2). The result is a system designed to control reflux, while achieving more than 70% coverage of the target structure.
The Neurochase Acute Drug Delivery System has a unique proprietary cannula design that allows the fluid to run back along the exposed length of the cannula up to the step. Furthermore, it has a soft, coated outer guide-tube design that prevents the fluid from travelling any further. This innovative approach is critical for achieving the intended distribution of volume deep within the brain tissue.
“BOTH PARTNERS EMPHASISED THE IMPORTANCE OF DESIGNING THE CLINICAL WORKFLOW TO BE HUMAN-ERROR-PROOF.”
DESIGNING OUT HUMAN ERROR: CLINICAL WORKFLOW FIRST
A core principle of the collaboration between Neurochase and Fearsome was ensuring that the device is intuitive and robust in the stressful environment of an operating room. Both partners emphasised the importance of designing the clinical workflow to be human-error-proof.
For the infusion phase, the system removes the necessity for manual programming of multiple pumps. Instead, a “dispenser” concept ensures that the drug is prepared and checked by the hospital pharmacist, enabling the technician or nurse to simply connect pre-labelled components (Catheter #1 to Dispenser #1, and so on) and start the infusions. This workflow-led design ensures that precision is baked into the system, reducing dependence on the surgeon’s real-time calculations and minimising human error.
THE OPERATING ROOM VERSUS THE DRAWING BOARD
Designing novel drug delivery systems forces a meeting between rigid engineering requirements and the dynamic realities of surgical practice. That meeting often produces productive, albeit challenging, tension. Developing highly complex medical devices requires rigorous documentation and controlled processes. Even small changes may need extensive paperwork, sign-offs and re-testing to meet the necessary regulatory and internal standards.
Creating innovative and useful solutions, in contrast, requires rapid and agile iteration – identify what failed, understand why and fix it. This is especially important when working without precedent and pushing into true innovation. Developers will always want to keep refining the device, but there is a process to go through and that is where the challenge lies. From an engineering perspective, there is a huge amount of work done to document progress, which can make quick pivots difficult.
At its heart, the friction is simple: surgical practice demands speed and adaptability; patient safety and regulators demand process and evidence. Resolving that tension requires a deliberate compromise. The Fearsome team balanced responsiveness with rigour by batching and gating changes. Surgical feedback was collected comprehensively but implemented only at defined design-review points and scheduled development cycles. This approach preserved iterative learning while producing the coherent, traceable body of evidence regulators require. By clearly defining when the drawing board could adapt to the operating room, the team kept their innovative momentum alive without sacrificing the discipline necessary for safe, compliant medical device development.
PRECLINICAL IMPERATIVE: THE COST OF NOVELTY
Because the Neurochase Acute Drug Delivery System represents a significant advance in the field of direct brain drug delivery, the team could not rely on existing benchmarks. Determining the performance bar for this novel device hinged on rigorous preclinical validation using in vivo. The team had to scrutinise why the device concept did or did not work and try to understand what was going on throughout the development process.
This constant feedback loop, which involved Fearsome engineers observing hundreds of live procedures, provided the essential physiological data needed to refine the system’s mechanics. The success of the system is a function of the mechanics being subservient to the physiology, ensuring that the device enables the desired clinical effect without causing harm. Unlike traditional models, where medical device engineers might meet with end users once or twice a year, the Neurochase and Fearsome collaboration enabled near-daily iteration. The engineering team observed and scrutinised every preclinical procedure, immediately feeding data back into the design process.
The collaboration between Neurochase and Fearsome is best described as a seamless, high-velocity fusion of deep clinical expertise and responsive, innovative engineering (Figure 3). The partnership’s unique structure is successfully tackling a novel and complex medical device challenge and is accelerating the path to life-changing neurological therapies.

Figure 3: A subset of the joint Fearsome

