To Issue 181
Citation: McLaughlin F, Edbrooke M, “Improving RNA Therapeutic Targeting Beyond Delivery”. ONdrugDelivery, Issue 181 (Dec 2025), pp 56–59.
Dr Fiona McLaughlin and Dr Mark Edbrooke discuss the possibilities for developing novel cancer treatments using functionalised nanoparticles in order to transport a variety of therapies into the tumour microenvironment.
In oncology, the low therapeutic index of traditional chemotherapy drugs poses a persistent barrier – achieving cytotoxic concentrations in tumour cells often results in systemic toxicity that limits efficacy. The rise of ribonucleic acid (RNA) therapeutics, comprising antisense oligonucleotides (ASOs), small interfering RNA (siRNA) and microRNA, offers a route to greater precision. Over the past five years, the number of US FDA-approved RNA therapeutics has risen sharply, yet all currently approved drugs of this class involve either “local” delivery to the central nervous system or only target a single organ – the liver – because no clinically validated platform has yet achieved safe, targeted delivery elsewhere in the body.1
“THE CHALLENGE NOW IS TO DIRECT THESE POWERFUL MOLECULES ACCURATELY TO THEIR INTENDED CELLULAR TARGETS BEYOND THE LIVER, PARTICULARLY IN COMPLEX SOLID TUMOURS.”
The “genome era” has enabled researchers to map disease-causing mutations, spurring the development of personalised nucleic acid drugs capable of silencing or modulating these mutations. The challenge now is to direct these powerful molecules accurately to their intended cellular targets beyond the liver, particularly in complex solid tumours.
BEYOND SINGLE-TARGET DELIVERY
Recent research emphasises that multi-targeting and dual loading are the next transformative steps in cancer treatment.2,3 If RNA therapeutics can be delivered selectively not only to tumour cells, but also to the cells of the surrounding tumour microenvironment (TME), such as fibroblasts, macrophages or T-cells, then multiple disease pathways could be modulated simultaneously. Furthermore, co-delivering two distinct cargos (for example dual siRNAs or an siRNA with a chemotherapeutic) could notably increase therapeutic precision and overcome resistance mechanisms.
Nanoparticle-mediated active targeting strategies, such as arginyl-glycyl-aspartic acid (RGD)-functionalised particles that recognise integrins overexpressed in the TME, offer substantial improvements to therapeutic index and delivery accuracy. These targeted systems overcome the limitations of relying on passive accumulation via the enhanced permeability and retention effect, and enable selective delivery to stromal and immune cells within tumours. Functionalised nanoparticles also exhibit stimuli-responsive features, such as pH-triggered release in the acidic TME, enhancing localised cargo delivery and minimising systemic exposure.
OVERCOMING CURRENT CHALLENGES
An example of the challenge of drug delivery is illustrated by pancreatic carcinoma where up to 90% of the tumour mass consists of cancer-associated fibroblasts (CAFs) and only 10% of malignant epithelial cells (Figure 1).5 The dense extracellular matrix produced by activated CAFs acts as a physical barrier to conventional drug penetration, whether they are small molecules or biologics. Similarly, immunotherapies struggle because the fibrotic and hypoxic microenvironment restricts immune cell infiltration. Overcoming this requires systems that can target both tumour cells and stromal elements, ideally delivering distinct but complementary cargos.
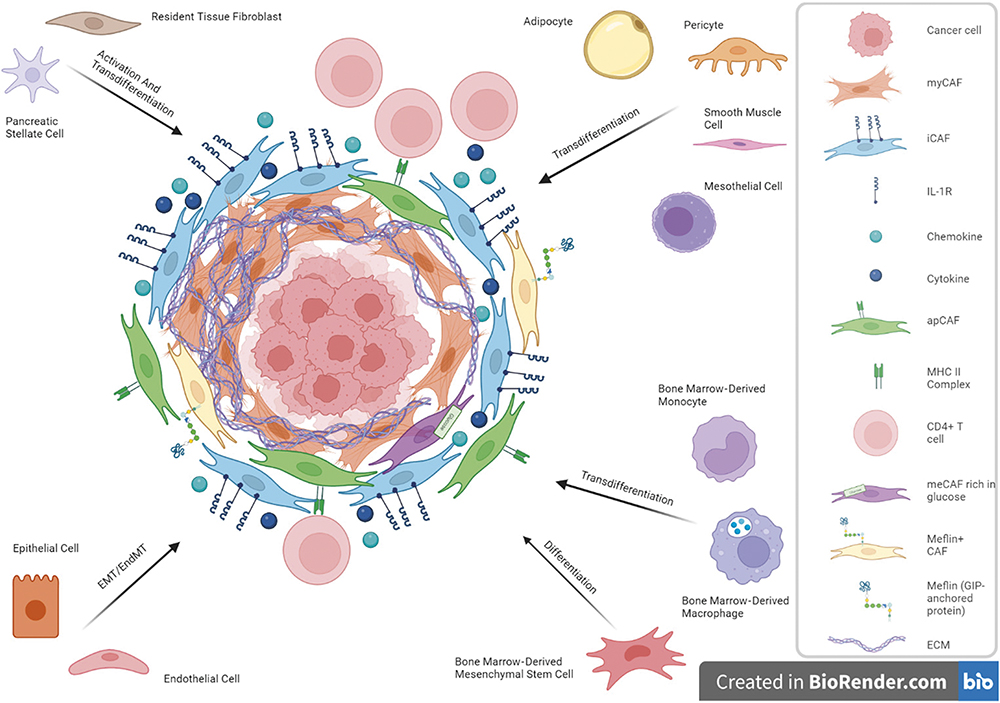
Epithelial-mesenchymal transition / endothelial-mesenchymal transition (EMT/EndMT), myofibroblastic CAF (myCAF), inflammatory CAF (iCAF), interleukin-1 receptor (IL-1R), antigen-presenting CAF (apCAF), major histocompatibility complex Class II (MHC II), metabolic CAF (meCAF), extracellular matrix (ECM).
Figure 1: A diagram illustrating CAFs and cellular origin heterogeneity in pancreatic ductal adenocarcinoma.4
“EARLY PRECLINICAL PLATFORMS SUCH AS SILICA-BASED NANOPARTICLES ARE NOW BEING ENGINEERED SPECIFICALLY FOR THIS MULTIFACETED TARGETING, PROVIDING A SOPHISTICATED BRIDGE BETWEEN NANOTECHNOLOGY AND TUMOUR BIOLOGY.”
Beyond chemical modifications to improve nanoparticle stability, the use of bispecific ligands targeting both CAF-associated proteins and integrins significantly enhances tumour uptake and therapeutic efficacy. This dual-targeting approach is central to tackling stromal barriers, which traditional drugs cannot cross efficiently. Early preclinical platforms such as silica-based nanoparticles are now being engineered specifically for this multifaceted targeting, providing a sophisticated bridge between nanotechnology and tumour biology.
ONE PAYLOAD IS NOT ENOUGH
Tumour heterogeneity and adaptive resistance limit the long-term efficacy of single-agent therapies. Combination or multi-drug delivery systems allow simultaneous modulation of different cancer pathways and phenotypes.3
For instance, systems that can carry multiple cargos, such as siRNAs targeting both epidermal growth factor receptor and polo-like kinase 1 in non-small cell lung cancer, have demonstrated improved inhibition and reduced compensatory resistance.6 Similarly, co-loading chemotherapeutic and RNA agents within a single carrier enables synergistic control of tumour growth and immune modulation. PEGylation and surface functionalisation further improve circulation and protect therapeutic cargo from proteolytic degradation en route to their targets.
DIFFERENTIATING TARGETS IN ONCOLOGY
Recent studies using silica nanoparticle-based delivery systems demonstrate how scalable, precision-engineered platforms can be fine-tuned for multi-target approaches. Advances in surface functionalisation, including the attachment of antibodies, ligands or aptamers, enable nanoparticles to recognise specific receptor patterns across tumour and non-tumour cells in the TME (Figure 2).
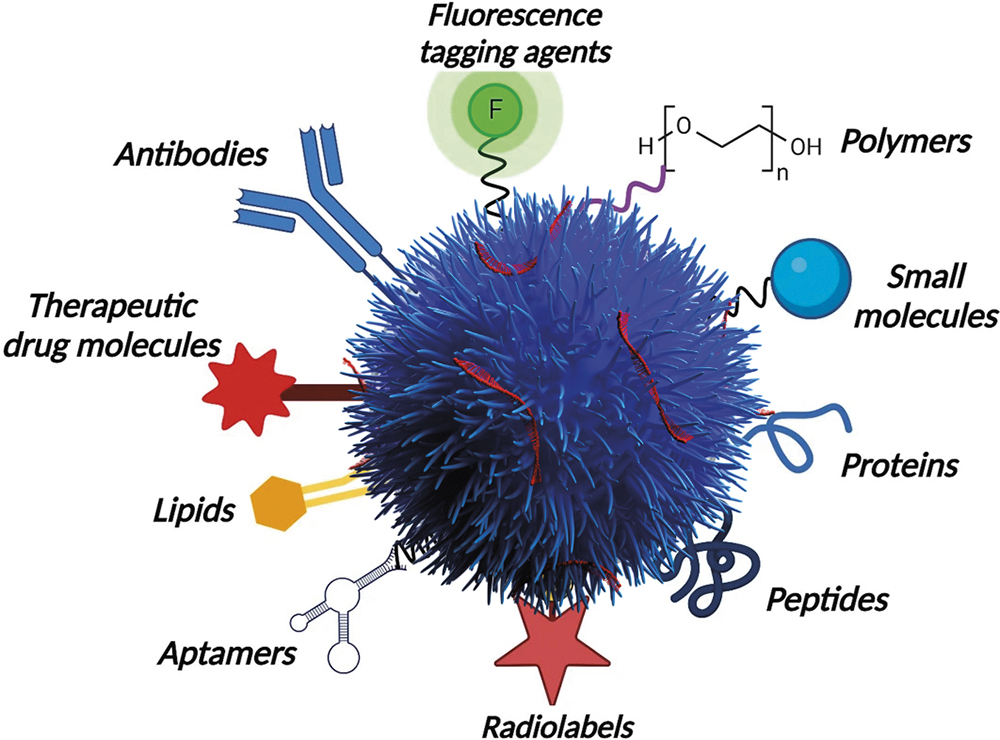
Figure 2: A diagram illustrating how Nuvec® (N4 Pharma) silica nanoparticles can be functionalised to deliver multiple therapeutic and diagnostic agents.
A mesoporous silica-based nanoparticle can deliver siRNAs or small molecules to multiple cell types simultaneously, ensuring deeper tissue penetration and balanced modulation of tumour biology. For example, functionalisation with an αvβ6 ligand achieved precise RNA delivery into epithelial tumour cells, including lung, breast, prostate and pancreatic adenocarcinomas. These targeted systems can achieve selective uptake, confirming that they can be steered to specific cancer subtypes, a major advance over non-functionalised platforms. This multi-receptor approach offers a translational pathway to address tumour heterogeneity, validated by significant progress in the development of preclinical lung and pancreatic models.
AMPLIFYING SUCCESS
The promise of dual-cargo, multi-target nanoparticle therapies lies in their modularity. In principle, delivery platforms can:
- Load different modalities (small molecules, oligonucleotides or peptides) tuned for specific release profiles
- Target multiple cell types, whether malignant, stromal or immune, by incorporating diverse ligands on its surface
- Exhibit environment-sensitive release, responding to the acidity, redox conditions or enzyme composition of the TME to ensure localised administration for systemic use.
These attributes align directly with the evolving commercial need for precision RNA therapeutics. With scalable manufacturing and predictable safety profiles, silica nanoparticle carriers could enable precise delivery of siRNA or ASO drugs to previously inaccessible tissues, extending the use of RNA therapeutics beyond hepatic models to complex cancers. Notably, studies from Lorenzoni et al stress the need for harmonised physico-chemical characterisation, toxicity evaluation and manufacturing standards to ensure clinical translation of such advanced nanoparticles.2 Collaborative frameworks between researchers, clinicians and regulatory bodies will be vital for success.
“SILICA NANOPARTICLE CARRIERS EXEMPLIFY THE NEXT EVOLUTIONARY STEP TOWARDS SCALABLE, PRECISE AND ADAPTABLE DELIVERY SYSTEMS FOR RNA AND COMBINATION ONCOLOGY THERAPEUTICS.”
CONCLUSION
The convergence of dual loading and multi-targeting provide a practical pathway towards overcoming the two greatest obstacles in oncology therapeutics: tumour heterogeneity and microenvironmental resistance. As highlighted by the emerging literature on RGD-functionalised systems, precision nanoparticle delivery can orchestrate simultaneous engagement of multiple pathways across the tumour ecosystem, transforming how efficacy, safety and resistance of these therapies are balanced.
Silica nanoparticle carriers exemplify the next evolutionary step towards scalable, precise and adaptable delivery systems for RNA and combination oncology therapeutics. While technical and translational challenges remain, such as protein corona formation and patient-specific microenvironment variability, the strategic integration of targeting ligands and multiplexed cargos offers a roadmap towards more effective, less toxic cancer treatments, redefining the therapeutic landscape for complex malignancies such as pancreatic and lung cancer.
By integrating adaptive design principles from nanotechnology with precision targets derived from molecular oncology, silica nanoparticle systems can transform how RNA and combination therapies are deployed.
REFERENCES
- Jo SJ et al, “Clinical pharmacokinetics of approved RNA therapeutics”. Int J Mol Sci, 2023, Vol 24(1), p 746.
- Lorenzoni S, Rodríguez-Nogales C, Blanco-Prieto MJ, “Targeting tumor microenvironment with RGD-functionalized nanoparticles for precision cancer therapy”. Cancer Lett, 2025, Vol 614, art 217536.
- Imtiaz S et al, “Mechanistic study of cancer drug delivery: current techniques, limitations, and future prospects”. Eur J Med Chem, 2025, Vol 290, art 117535.
- Saúde-Conde R et al, “Cancer-associated fibroblasts in pancreatic ductal adenocarcinoma or ametaphor for heterogeneity: from single-cell analysis to whole-body imaging”. Biomedicines, 2024, Vol 12(3), p 591.
- Domen A et al, “Cancer-associated fibroblasts as a common orchestrator of therapy resistance in lung and pancreatic cancer”. Cancers, 2021, Vol 13(5), p 987.
- Chai M et al, “Targeted and intelligent nano-drug delivery systems for colorectal cancer treatment”. Front Bioeng Biotechnol, 2025, Vol 13, art 1582659.

