To Issue 181
Citation: Beike H, Heine B, “The Importance of Mesh Nebuliser Configuration in mRNA-LNP Delivery Performance”. ONdrugDelivery, Issue 181 (Dec 2025), pp 24–27.
Hanna Beike and Dr Benjamin Heine highlight the superior performance of customisable drug-specific nebuliser platforms, such as eFlow® Open System and eFlow® Integrated, for the delivery of messenger RNA-based inhalation therapies.
INHALABLE mRNA FOR THE TREATMENT OF RESPIRATORY DISEASE
During the covid-19 pandemic, the use of messenger RNA (mRNA) encapsulated in lipid nanoparticles (LNPs) came to the fore as new mRNA vaccines were rapidly developed, approved and rolled out in large-scale vaccination programmes. During this time, the preclinical development of inhalable mRNA vaccines was initiated, in addition to the more commonly used intramuscular injection delivery method.
In fact, inhalable mRNA was already being investigated prior to the pandemic as a potential therapy for respiratory disease, with the direct route of inhalation providing faster therapeutic effect, while reducing the extent of systemic drug exposure.
Today, numerous research programmes are underway with the aim of using inhalable mRNA therapies to treat various lung diseases including, among others, cystic fibrosis (CF), lung cancer and primary ciliary dyskinesia, with some promising early results.1–4 For example, although not yet proven to be efficacious, interim results from a randomised placebo-controlled Phase I/II trial have shown that MRT5005 – an investigational codon-optimised cystic fibrosis transmembrane conductance regulator (CFTR) mRNA developed for treating CF and delivered by nebulisation – is generally safe and well tolerated in adults with two severe Class I and/or II CFTR mutations.1
“NEBULISATION OF mRNA FORMULATIONS ENCAPSULATED IN LNPs PRESENTS SEVERAL TECHNICAL AND BIOLOGICAL CHALLENGES, INCLUDING PHYSICAL STABILITY OF mRNA MOLECULES AFTER NEBULISATION,
FILL VOLUME AND DELIVERY EFFICIENCY,
WHICH NEED TO BE CONSIDERED WHEN SELECTING THE APPROPRIATE NEBULISER.”
MESH NEBULISERS ARE A PREFERRED CHOICE FOR TREATING SEVERE RESPIRATORY DISEASES
Nebulisation of mRNA formulations encapsulated in LNPs presents several technical and biological challenges, including physical stability of mRNA molecules after nebulisation, fill volume and delivery efficiency, which need to be considered when selecting the appropriate nebuliser. Today, mesh nebulisers are the preferred choice when treating severe respiratory diseases,5 and this also applies for nebulisation of mRNA.
The gentle aerosolisation mechanism of mesh nebulisers compared with other nebuliser technologies, such as ultrasonic or jet nebulisers, and the associated high delivery efficiency, favour mesh technology. The ability to better accommodate low-concentration drug formulations due to a large reservoir is an additional advantage, specifically over soft mist inhalers. This is because the latter allow only a few microlitres per puff and therefore require higher concentrations, which could limit formulation development and stability of the API. Another benefit is the ability of mesh nebulisers to deliver aerosolised formulations during normal tidal breathing, which helps to enhance patient comfort and ease of use.
Recent advances in mesh nebuliser technology have also significantly enhanced drug delivery precision and efficiency. Breath-triggered systems enable aerosol generation exclusively during stages of the inspiratory phase, thereby optimising dosing accuracy and minimising drug loss. Historically, these systems were limited by prolonged nebulisation times. However, innovations in mesh design have substantially increased aerosol output rates and, along with an innovative breath-guiding concept,6 effectively overcome this limitation.
As the following study demonstrates, the customisation of nebulisers for ideal drug delivery is an essential process. Customisation enables the correct amount of the intact API to be delivered, resulting in levels of cellular uptake and bioavailability sufficient for therapeutic efficacy.
mRNA DELIVERY PERFORMANCE OF DIFFERENT MESH NEBULISERS
A recent study compared the performance of different mesh nebulisers in the delivery of LNP-encapsulated mRNA, analysing the preservation of mRNA integrity in an indirect method, degree of drug delivery and resulting lung deposition for each device.7
Five vibrating mesh nebulisers were evaluated, including the drug-specific nebulisers eFlow® Integrated6 and eFlow® Open System Type A and B (eFlow OS A/B) as well as two general-purpose devices; eFlow® rapid and Aerogen® (Galway, Ireland) Solo (Table 1). The eFlow OS A and B differ in hole geometry and the number of holes laser-drilled into the mesh. For drug-specific nebulisers, the laser-drilling parameters and therefore the hole geometry can be adapted to the drug, which is crucial for complex formulations such as LNP suspensions.
| Nebuliser | MMAD (μm) | TOR (mg/min) | GSD | DD (%) |
| eFlow Integrated | 5.2 ± 0.2 | 1568 ± 31 | 1.8 ± 0.0 | 96.4 ± 2.1 |
| eFlow OS A | 5.4 ± 0.2 | 1223 ± 66 | 1.9 ± 0.0 | 50.9 ± 1.6 |
| eFlow OS B | 3.9 ± 0.1 | 1057 ± 16 | 1.5 ± 0.1 | 65.6 ± 2.5 |
| eFlow rapid | 4.8 ± 0.1 | 1168 ± 76 | 1.6 ± 0.1 | 28.2 ± 1.0 |
| Aerogen Solo | 4.3 ± 0.3 | 356 ± 27 | 1.9 ± 0.1 | 38.9 ± 1.3 |
DD = delivered dose; GSD = geometric standard deviation; MMAD = mass median aerodynamic diameter; TOR = total output rate.
Table 1: Nebulisers used in the study including aerosol and performance data (ISO 24724).
Intracellular protein expression was measured in vitro for both pre- and post-nebulised mRNA-LNP formulations. Device performance was evaluated using 2.5 mL of Sultanol® (salbutamol) forte (GlaxoSmithKline) and lung deposition was analysed through multiple-path particle dosimetry (MPPD) modelling.8
The eFlow Integrated Device Demonstrates Superior Drug Delivery
The analysis of delivered dose (DD) – the first hurdle of the delivery cascade – showed that the breath-triggered eFlow Integrated device was clearly superior with a DD of 96.4% compared with the other four continuous nebulisation devices, whose DD ranged from 28.2% to 65.6% (Figure 1a & Table 1).
Importantly, the difference in DD observed between the general-purpose eFlow rapid and customisable eFlow OS devices was driven not only by differences in mass median aerodynamic diameter but also by other device customisation. For example, the smaller aerosol chamber and the residual volume in the reservoir of eFlow rapid deliberately reduces the DD to prevent overdosing of drug products developed for administration in general-purpose nebulisers, thus reiterating the importance of device customisation to maximise performance for delivery of novel inhaled drug products.
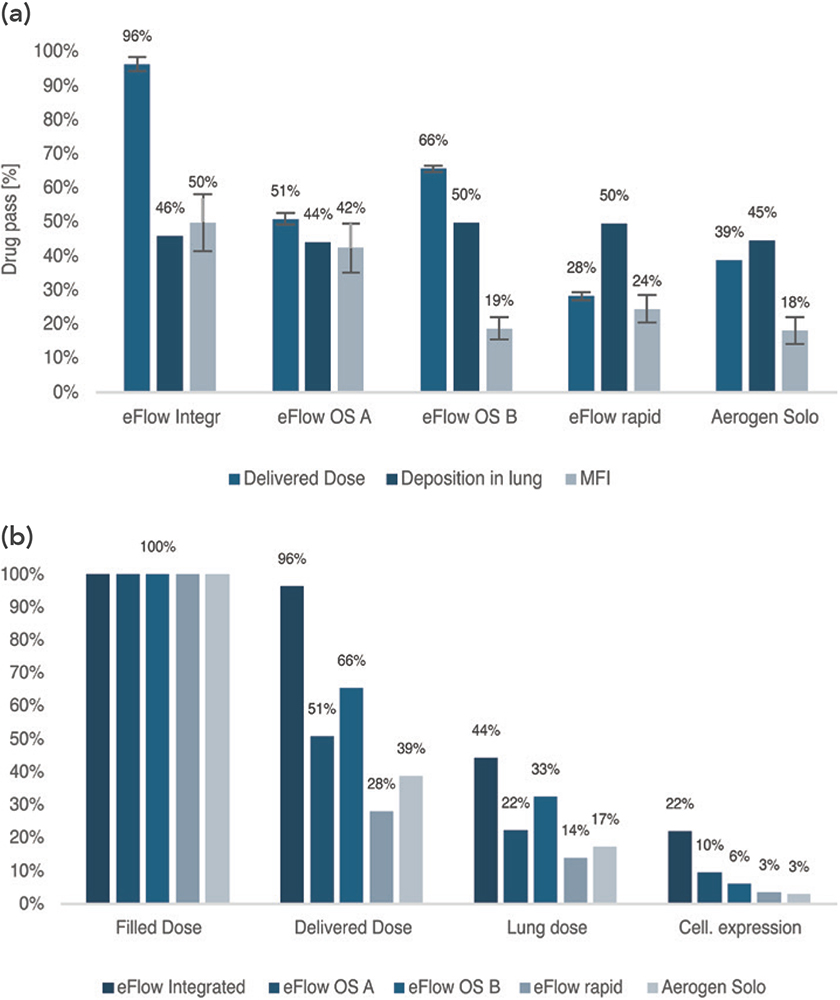
Figure 1: (a) Fraction of drug passing each of the three hurdles of the delivery cascade (b) Cascade of usable mRNA throughout the delivery process.
Measures of Lung Deposition are Comparable Across Devices
The second hurdle of the delivery cascade depends on the fraction actually delivered to the lung. While the degree of lung deposition estimated by the MPPD model did not vary greatly among devices (Figure 1a), ranging from 44% (eFlow OS A) to 50% (eFlow OS B, eFlow rapid), it should be noted that lung doses for the eFlow devices are likely to be underestimated. For example, for both the eFlow OS A/B and eFlow rapid, the aerosol bolus generated by the aerosol chamber led to transient aerosol output, which reduces exhalation losses and increases lung deposition.9 Similarly, the trigger algorithm of the eFlow Integrated device halts nebulisation before the end of inspiration to minimise exhalation losses.6 These effects are not accounted for in the MPPD model and can therefore result in underestimated lung doses.
The eFlow OS A and eFlow Integrated Nebulisers Elicit Higher Levels of Protein Expression
The third hurdle of the delivery cascade assessed the drug’s intracellular activity, quantified by mean fluorescence intensity (MFI) of transfected cells. This analysis of protein expression served as an indicator of mRNA-LNP integrity following nebulisation and was compared with a pre-nebulisation sample across all five devices.
The lowest levels of protein expression (all <25% MFI compared with the non-nebulised sample) were observed with the Aerogen Solo, eFlow OS B and eFlow rapid (Figure 1a), indicating that a substantial portion of the nebulised mRNA failed to induce protein expression. In contrast, the highest expression values were obtained for eFlow OS A and eFlow Integrated (42% and 50% MFI, respectively), demonstrating greater post-nebulisation mRNA-LNP integrity and thus more effective nebulisation with these devices.
The in vitro protein expression analysis was selected since it offers greater insight into therapeutic efficacy than other parameters such as hydrodynamic diameter or encapsulation efficiency, capturing intracellular activity rather than just LNP characteristics.
In Vitro Cellular Protein Expression is Greatest with the eFlow Integrated Device
Considering all three hurdles of the delivery cascade, the delivery efficiency in terms of in vitro cellular protein expression can be determined (Figure 1b). The eFlow Integrated nebuliser emerged as superior, achieving levels of cellular protein expression (22%) that were over double the degree of expression achieved by the other devices. Of all the nebulisers tested, the eFlow Integrated was therefore the most effective for delivering LNP-encapsulated mRNA, followed by eFlow OS A and B. The general-purpose devices eFlow rapid and Aerogen Solo achieved the lowest levels of cell expression (3%), a finding that was also observed by van Rijn et al (2023).10 In the present study, tailoring the nebuliser to the drug led to a seven-fold increase in cell expression. This finding emphasises the importance of customisation.
The eFlow Integrated Nebuliser Exhibits Rapid Drug Delivery Times
With the results of protein expression (equivalent to 1,000 mg of drug product in the lung cell) and total output rate (TOR), drug delivery times can be calculated. When comparing all five devices, the eFlow Integrated achieved the shortest delivery time of just 5.8 minutes, achieved due to high efficiency and high TOR. The longest delivery time of 90.3 minutes was found for the Aerogen Solo, reflecting low efficiency and TOR (Table 2).
| eFlow Integrated | eFlow OS A | eFlow OS B | eFlow rapid | Aerogen Solo | |
| TOR (mg/min) | 1568 | 1223 | 1057 | 1168 | 356 |
| Cell Expression | 22% | 10% | 6% | 3% | 3% |
| Delivery Time (min) | 5.8 | 8.6 | 15.6 | 25.0 | 90.3 |
Table 2: Calculated nebulisation time for an identical cell dose (1,000 mg drug product) for each nebuliser. For the triggered device the theoretical maximum duty cycle (head-on:head-off time) of 50% for the standard breathing pattern was assumed.
CONCLUSION
These findings suggest that it is crucial to select an appropriate nebuliser and configuration to achieve effective nebulisation of LNP-encapsulated mRNA and a resulting optimal cellular response.
While the performance of general-purpose nebulisers not intended for mRNAs is suboptimal, customisable drug-specific product platforms, including the eFlow OS and eFlow Integrated, demonstrate superior performance for mRNA-based inhalation therapies, resulting in highly efficient drug delivery and mRNA preservation.
The eFlow OS A and the breath-triggered eFlow Integrated nebuliser are effective for the delivery of LNP-encapsulated mRNA, indicating potential therapeutic efficacy. Nebulisation with the eFlow Integrated resulted in the highest levels of DD and in vitro cellular protein expression, along with displaying the fastest drug delivery time compared with the other nebulisers.
REFERENCES
- Rowe SM et al, “Inhaled mRNA therapy for treatment of cystic fibrosis: Interim results of a randomized, double-blind, placebo-controlled phase ½ clinical study”. J Cyst Fibros, 2023, Vol 22(4), pp 656–664.
- Hu B et al, “Modulating tumor collagen fiber alignment for enhanced lung cancer immunotherapy via inhaled RNA”. Nat Comm, 2025, Vol 16, art 8120.
- Hennig M et al, “Inhaled DNAI1 mRNA therapy for treatment of primary ciliary dyskinesia”. Proc Natl Acad Sci USA, 2025, Vol 122(18), art e2421915122.
- Sarode A et al, “Inhalable dry powder product (DPP) of mRNA lipid nanoparticles”. Drug Deliv Trans Res, 2024, Vol 14(2), pp 360–372.
- Pritchard J et al, “Mesh nebulizers have become the first choice for new nebulized pharmaceutical drug developments”. Ther Deliv, 2018, Vol 9(2), pp 121–136.
- Braeunlich G, Hahn M, “Deep Breaths, Deep Dives: The New eFlow Integrated Nebuliser”. ONdrugDelivery, Issue 158 (Apr 2024), pp 16–19.
- Beike H et al, “Analysis of mRNA Delivery Performance via Vibrating Mesh Nebulization”. Conference Proceedings, Drug Delivery to the Lungs, Volume 36, 2025.
- Miller F et al, “Improvements and additions to the Multiple Path Particle Dosimetry model”. J Aerosol Sci, 2016, Vol 99(13), pp 14–26.
- Grill M et al, “Steady or not? Using realistic, transient aerosol parameters to predict lung deposition”. Conference Proceedings, Drug Delivery to the Lungs 2023, accessed online, Nov 2025.
- van Rijn C et al, “Low energy nebulization preserves integrity of SARS CoV 2 mRNA vaccines for respiratory delivery”. Sci Rep, 2023, Vol 13(1), p 8851.

