To Issue 140
Citation: Cuevas Brun EH, Hsu Y-M, “A Roadmap for Drug-Nebuliser Combination Product Development”. ONdrugDelivery, Issue 140 (Nov 2022), pp 54–58.
Edgar Hernan Cuevas Brun and Yuan-Ming Hsu lay out the HCmed Innovations’ roadmap for the development of drug-nebuliser combination products.
“A surge in the number of novel inhaled biologics has also directed focus towards the use of mesh nebulisers as one of the primary delivery platforms.”
To ensure the efficient drug delivery of inhaled formulations, extensive research and development of drug-device combination products is required to address diverse aspects from both elements. Research has shown that accurate and efficient delivery of an API to the correct airway section is as important as its dosage and efficacy, making delivery a crucial factor for the success of an inhaled product.
In recent years, the inhalation therapy field has seen an increasing demand for combination products when it comes to the use of nebulisers. This demand, driven by requirements for predictable and consistent delivery performance in particular, has received further stimulus from regulatory authorities, which has promoted updated guidelines to incentivise the development of combination products. Moreover, a surge in the number of novel inhaled biologics has also directed focus towards the use of mesh nebulisers as one of the primary delivery platforms.1
As biologic drugs are more susceptible to losing their therapeutic effect when exposed to hazardous conditions, such as high shear forces and high temperature, mesh nebulisers have been recognised as suitable delivery platforms. Mesh technology is commonly associated with only a minimal increase in temperature and shear force exposure when aerosolising liquid formulation.2
As a result of this increased demand, the necessity for a clear understanding of drug-nebuliser combination products has paved the way for the establishment of contract development and manufacturing organisations (CDMOs) that can support pharmaceutical partners with their desired expectations for device performance and the development and the selection of appropriate formulations and nebuliser platforms for the development of new inhaled treatments.
“The addition of appropriate excipients can support the aerosolisation of formulations, avoiding undesirable reactions that could otherwise lead to coughing or irritation of the airways.”
DRUG-NEBULISER COMBINATION PRODUCTS
The realisation of a drug-nebuliser combination product is the result of harmonising both elements. The liquid formulation and the nebuliser device both contribute to the expected therapeutic effect; therefore, optimisation of their interaction is critical. On the formulation side, understanding the physicochemical properties of the liquid, which could be either a solution or a suspension, is part of the background information required to initiate the combination pairing. Regarding delivery with mesh nebulisers, high viscosity and very low surface tension can influence the delivery rate.3 Furthermore, the involvement of biologic drugs introduces additional concerns, such as their stability and activity post-nebulisation, which could be significantly reduced when aggregation or structural unfolding occurs.
The selection of excipients plays another important role on the formulation side. The addition of appropriate excipients can support the aerosolisation of formulations, avoiding undesirable reactions that could otherwise lead to coughing or irritation of the airways. The purpose of these excipients extends from modifying the properties of the formulation to protecting the API. It has been documented that small amounts of polysorbates can be used to decrease the viscosity of a formulation, while lysine and arginine can be used to tailor aerosol characterisation performance.4
Alternatively, customisable mesh nebulisers, such as the AdheResp platform from HCmed Innovations, allow a wide range of modifications, including hardware and firmware. Hardware components can be modified to satisfy requirements related to loaded volume, indicators, device structure, user interface, connectivity and even the mesh component itself. This last one permits the adjustment of aerosol characterisation performance to identify the most suitable mesh membrane for the liquid formulation in the combination product. Similarly, the firmware can also be modified to define aerosolisation periods that are more effective for a specific formulation, as well as the delivery needs of a specific patient population or user behaviour requirement. Moreover, enabling and disabling Bluetooth and activation functions based on the requirements for the combination product enhances the flexibility of the platform firmware (Figure 1).
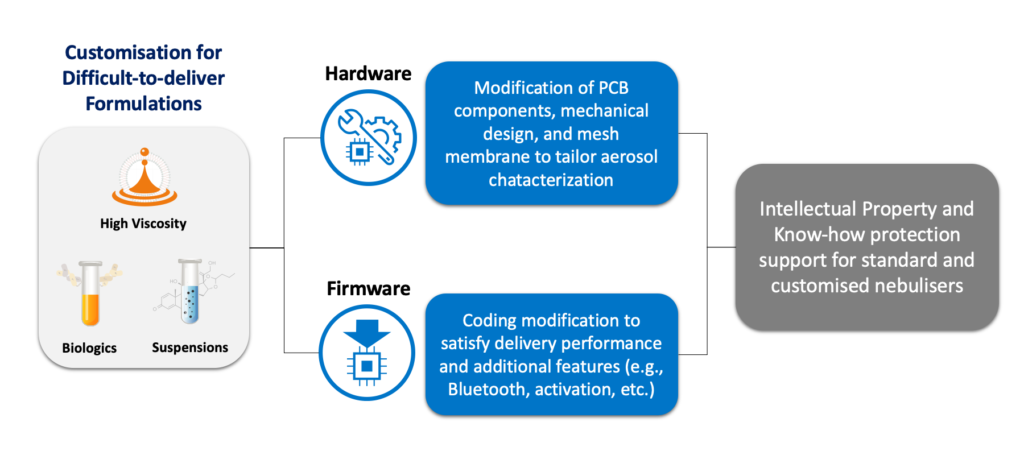
Figure 1: Device customisation scope for drug-nebuliser combination development.
Ultimately, the optimal matching of the nebuliser and formulation factors can define the efficacy of the final combination product.
“By generating data, assessing performance and customising the platforms in parallel, a reduction in cost and time is attainable.”
DRUG-NEBULISER COMBINATION CDMO
The scope for combination product development begins prior to a feasibility study. The value of a CDMO working on drug-nebuliser development can be clearly seen at the initial stage when comparing direct assessments of over-the-counter nebulisers with customisable platforms. While the former is only likely to provide in vitro data of the tested nebulisers, the latter could provide a comprehensive set of data with points to optimise delivery performance in the following stages, guaranteeing a higher success rate, and thus offering a more methodical approach.
At HCmed Innovations, which is focused on the development of drug-nebuliser combination products, the entire development is a well-rounded process that provides support to pharmaceutical partners at all stages, from preclinical and clinical stages to commercialisation (Figure 2) – a fully integrated path supported by HCmed’s proprietary mesh technology.
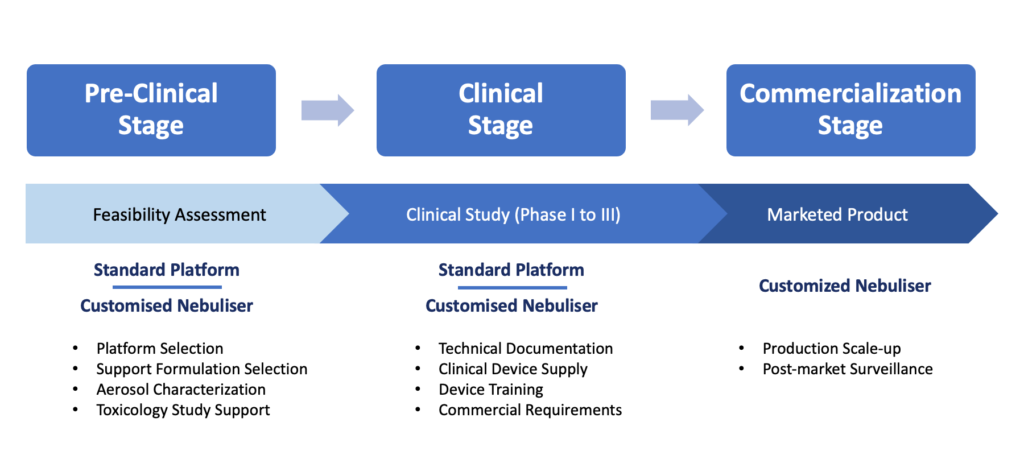
Figure 2: Drug-nebuliser combination CDMO services throughout the development stages.
The expectations for the development at each stage could be summarised as follows:
Preclinical Stage
Nebulisation of formulations can vary significantly due to each formulation’s distinctive properties. Furthermore, the requirements of aerosol characterisation (e.g. mass median aerodynamic diameter, fine particle fraction, geometric standard deviation) and delivered dose are equally important, as they are selected based on the indication (e.g. chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis, cystic fibrosis, pulmonary arterial hypertension, etc.) to be treated and the patient population. It is for this reason that selecting the correct platform from an early stage can greatly facilitate the development process.
The two main categories of mesh nebuliser platform can be classified as continuous output and breath-actuated. As their names suggest, a continuous output nebuliser generates aerosol continuously throughout the whole treatment, whereas a breath-actuated device aerosolises the formulation based on a triggering mechanism that allows generation during a specific phase of inhalation.
Depending on the requirements for delivery, it would be expected that treatments that prioritise delivery time over a high-dose delivery would be more suitable for a continuous output mode platform. Conversely, a breath-actuated nebuliser would be the preferred choice when prioritising the delivered dose and reduction of fugitive aerosols.
The user interface also plays a major role when establishing design requirements, such as user feedback. For that reason, distinguishing these requirements at an early stage is helpful for ensuring smooth project development. Furthermore, the security provided by a strong intellectual property portfolio for standard and customised nebulisers, along with the corresponding freedom to operate in the selected countries and territories, enhances the value of the device for the combination product.
Once the platform is selected, an understanding of the formulation and nebuliser interaction is indispensable, and it is here that a supportive team of experienced professionals in the aerosol delivery field is essential. The team at HCmed Innovations, which is formed of experts in the field, can offer suggestions that extend beyond device customisation to the point of tapping into formulation comprehension. The benefit then lies in recommending a customisable version of the device based on the pre-formulation assessment and, therefore, assisting with the formulation selection and the most suitable nebuliser.
To support the selection and testing process, HCmed works with a state-of-the-art in vitro laboratory that is equipped with analytical capabilities and a group of testing engineers and knowledgeable scientists familiar with the nebulising platforms used. Aerosol characterisation via laser diffraction particle size analysers and cascade impactors, as well as delivered dose assessment with breath simulators, are some of the studies that HCmed conducts at the development stage. By generating data, assessing performance and customising the platforms in parallel, a reduction in development cost and time is attainable. Moreover, HCmed’s installations not only cover testing of continuous mode devices but also of breath-actuated platforms with the addition of mixing inlets, which can help accelerate initial development stages. Last but not least, for biologic drugs, the aerosol performance analysis of a biologic compound’s stability and activity post-nebulisation must also be covered within the scope of the feasibility study.
To complete the activities of the preclinical stage, toxicology research is also performed, at which point receiving support for the toxicology study setup is extremely valuable.
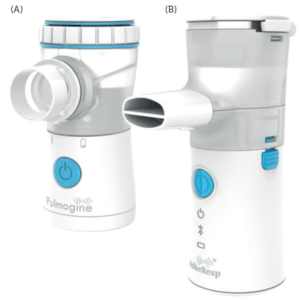
Figure 3: (A) Pulmogine vibrating mesh nebuliser (continuous output mode). (B) AdheResp
smart breath-actuated mesh nebuliser (breath-actuated mode).
Clinical Stage
When entering the clinical stage, device documentation compliant with the regulatory standards of different health authorities plays a major role. Although standard platforms come with corresponding technical documentation packages, newly customised nebulisers also require equivalent documentation.
The HCmed team ensures that the device development process complies with all the aspects needed to satisfy regulatory requirements, including device risk assessment, chemical risk assessment, process failure mode effects analysis, design failure mode effects analysis, device verification and validation documentation, among others. This process guarantees the safety and efficacy of the nebuliser, which is part of the requirement for the clinical trial applications. Moreover, GMP-compliant device supply and training for each trial are part of HCmed’s services.
In some instances, the use of standard platforms, such as HCmed’s Pulmogine (continuous output – Figure 3a) or AdheResp (breath actuated – Figure 3b), may be suggested for a Phase I trial, leaving the customised nebuliser for later clinical phases. This type of development strategy can ensure reliance on the same technology, which simplifies the transition to a customised version in later stages.
Consequently, collaborating with a trusted partner at the clinical stage is a critical point when taking into consideration the length and cost of clinical trials. This is the stage at which a company such as HCmed can provide strong support and reliability.
Commercialisation Stage Quality assurance for the production scale-up of the nebuliser is the pillar for the commercialisation stage, along with an already evaluated and guaranteed freedom-to-operate status. However, the work goes beyond this point and covers extensive post-market surveillance to monitor the performance of the marketed device and offer assistance on any issue that may arise from the use of the product. HCmed’s driven motivation and key goals as a CDMO partner are always to ensure patients’ comfort, safety and treatment efficacy for the drug-nebuliser combination product.
CONCLUSION
Recent advancements in inhalation therapy have created demand for the use of nebulisers in novel drug-device combination products, establishing a market for services that can be fulfilled by companies that provide a fully integrated development path. Although it is suggested that this path be adopted from early-stage development, its integration can bring wide-ranging benefits for pharmaceutical companies at any stage. The objective is to facilitate the development process and increase the likelihood of originating new treatment options for a significant number of diseases that could use the respiratory route to achieve the desired therapeutic effect.
To summarise, the development of a drug-nebuliser combination product is a lengthy process that requires constant interaction and input from both parties to tailor both the formulation and nebuliser, aiming for an optimal match. The role of a CDMO supporting this process is to provide a roadmap from conceptualisation to commercialisation to support pharmaceutical partners with experts at each stage, shortening development timelines and reducing development risks to create an optimal combination.
REFERENCES
- Fröhlich E, Salar-Behzadi S, “Oral inhalation for delivery of proteins and peptides to the lungs”. Eur J Pharm Biopharm, 2021, Vol 163, pp 198–211.
- Pritchard JN et al, “Mesh nebulizers have become the first choice for new nebulized pharmaceutical drug developments”. Ther Deliv, 2018, Vol 9(2), pp121–136.
- Cuevas Brun EH, Hsu Y-M, “Delivering Suspensions with Mesh Technology”. ONdrugDelivery, Issue 127 (Nov 2021), pp 38–41.
- Beck-Broichsitter M, “Making Concentrated Antibody Formulations Accessible for Vibrating-Mesh Nebulization”. J Pharm Sci, 2019, Vol 108(8), pp 2588–2592.

