To Issue 160
Citation: Gross C, Tunkel M, “Addressing Diabesity with Pen Injector Platforms”. ONdrugDelivery, Issue 160 (May 2024), pp 31–34.
Cécile Gross and Mark Tunkel discuss the variety of pen injector platforms to meet the different needs of diabetes patients.
Not a day goes by without reading in the newspapers an article on diabetes or obesity. A new word has also recently appeared: diabesity. It actually dates back to the 1970s when Ethan Sims originally used the term to describe studies in which healthy prisoners were deliberately overfed to gain weight and become overweight, but not obese. The aim was to demonstrate reversible rises in fasting glucose and a deterioration of glucose tolerance, not diabetes.
“Healthcare systems, buying powers and cultural habits differ between the US, Europe and India and will lead to differentiated offerings to meet patients’ needs.”
The former American Diabetes Association President Francine Kaufman, amongst others, has used this term since then to describe the co-existence of the twin pandemics – and the term spread. Exactly like the obesity pandemic itself. In 2022, the WHO estimated that one in eight people are living with obesity,1,2 showing a huge increase in adults and a massive increase in teenagers, which led to the acceleration of the WHO’s plan3 to stop it. This has been substantiated by a new study released by The Lancet4 – stating that more than 1 billion people in the world are living with obesity.
Diabetes is a worldwide health problem, and combining this condition with underweight or obesity adds to the burden on patients even more. This reflects the intricate relationship between complex chronic diseases and their snowball effects in today’s world.

Figure 1: Patient journey research to better understand a specific patient population, tailoring solutions to answering the unmet needs.
ARE ALL PATIENTS THE SAME?
Looking at available treatments for diabetes, insulin has been known and used for more than 100 years and, more recently, peptides such as glucagon-like peptide 1 (GLP-1) have also been used. Since the first US FDA-approved GLP-1 in 2009, pharma companies have worked to reduce injection frequency, improve the drug delivery device and even offer diverse administration routes, such as oral delivery. Although limited to Type 2 diabetes, they offer patients the possibility of having less-frequent injections. Obesity patients can now benefit from them as an alternative to bariatric surgery (Figure 1).
However, patients cannot be considered as one homogenous group: there is a discrepancy in incidence between women and men, adults and teenagers, and geography is also adding to the complexity of care. As an example, healthcare systems, buying powers and cultural habits differ between the US, Europe and India and will lead to differentiated offerings to meet patients’ needs. As a drug delivery device manufacturer and combination product service provider, Nemera’s responsibility is to consider these characteristics and implement them into its devices.
US patients, for example, usually perform unit-dose injection with a disposable device. Given the relatively high volume per dose to be injected, it implies a spring-assisted injection. European patients still need to perform a high-volume injection, but they will use either a unit-dose or a multiple-dose disposable delivery device. In both cases, injection is spring assisted. Indian patients are very sensitive to cost per injection; therefore, they would rather use a multiple dose delivery device. Mostly they opt to use reusable devices as they are much more cost effective, whilst some others may still prefer to use manual disposable ones.
PEN INJECTOR PLATFORMS TO MEET DIFFERENT NEEDS
PenSET and PenDIA
The PenSET and PenDIA (Figure 2) platforms can accommodate GLP-1 drugs with a smooth spring-assisted injection. A multiple-dose device has been chosen rather than a unit-dose one to alleviate the burden on the patient. Having one month’s treatment available in one single device instead of four separate devices makes it less cumbersome, eases portability when travelling or going to work and fosters acquaintance with the device through longer use of the same device. The risk of dosing errors is also reduced as there is no possibility of overdosing during the four week period as the dose to be administered comes into the same device, not a separate one.
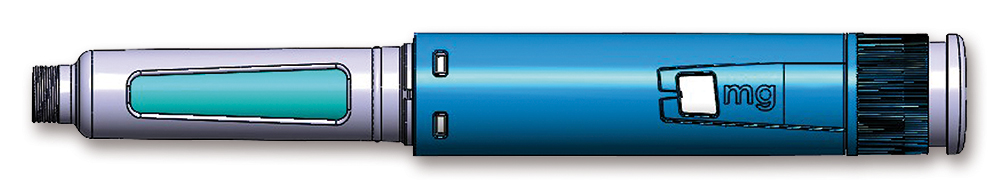
Figure 2: PenDIA – a spring-assisted pen injector for seamless GLP-1 administration.

Figure 3: PENDURA AD – a reusable pen injector that ensures the application of proven technological solutions with reliability and intuitiveness.
The reason why these two platforms coexist is to address the need for a fixed dose on one hand and a variable dose with dose correction on the other. In both cases, the result is fewer discarded devices in the end.
“When patients finish using the drug in the cartridge, they just have to replace it with a new one.”
PENDURA AD
PENDURA AD (Figure 3) is also a multiple-dose and spring-assisted platform; however, it is not disposable but reusable for three years. When patients finish using the drug in the cartridge, they just have to replace it with a new one. On top of being cost effective and offering a seamless injection, the device has several features to give patients a cue of injection dose completion: clear visibility of the drug, marking on the cartridge holder and a green dot once the device goes back to its initial position.
With a track record of more than 10 years in several markets, this platform has been selected by Nemera’s Danish partner Zealand Pharma. It is offering dasiglucagon, a next-generation ready-to-use glucagon analogue. Dasiglucagon is being developed for several therapies. Thanks to a constructive co-operation between the two companies, the PENDURA AD platform has been chosen to deliver dasiglucagon, and the benefits of this combination are being investigated in clinical trials.
On the topic of clinical trials, an integrated connected pen is also available. Customised from Nemera’s reusable pen platform PENDURA AD, it features a Bluetooth module integrated into the pen itself, which can help transfer data to an app. Before transferring it, the data related to the injected dose are displayed on the screen, together with the date and time of injection. Complete data are available in the history on the application.
PENVARIO
Nemera chose to develop a pen platform versatile enough to deliver the abovementioned drugs, as well as others. PENVARIO (Figure 4) is a manual pen platform able to address diabetes and obesity with rapid-acting or long-acting insulins and GLP-1, osteoporosis with parathyroid hormones, such as abaloparatide, and fertility with follicle stimulating hormones, such as follitropin alpha. Each variant of the platform has been designed to match the specificities of the different target populations and regimens.

Figure 4: PENVARIO – tailoring the injection with a variable fixed dose disposable platform.
Returning to the example of India, where the prevalence of diabetes is very high, a multidose, disposable manual pen makes the treatment affordable at a low pricing point. It also offers the same patient injection experience as the conventional insulin pen injector.
Another area of interest is to help keep track of the number of doses injected. When the treatment becomes regular, patients have difficulty following up their regimen. In addition, within the ageing population, a lot of people take more than one treatment frequently, so having to remember if they have taken their dose could be tricky. To avoid under dosing or overdosing, Nemera’s pen injectors can be customised with a specialised add-on. Designed to assist patients in monitoring their injections, it collects details on injected doses and adds them into a history. The aim is to accurately count the administered number of doses by recognising key parameters such as typical pen-use sequences, pen position and the distinctive injection-dose signature, while detecting each specific step of priming and injection.
INTEGRATED SERVICES AND MANUFACTURING CAPABILITIES TO SUPPORT THE DRUG-DEVICE COMBINATION
Partnering with Nemera can simplify the process of integrating the selected pen injector with drug products, accelerating the development of combination products and expediting time to market while managing the complexities associated with managing the process. The company ensures that the selected pen injector is tailored to the specific needs of patient populations, drug characteristics and regulatory requirements. Its comprehensive services and capabilities cover critical areas essential for the success of combination products, including:
- Analytical services and design verification
- Human factors and user experience management
- Instructional materials and packaging development
- Regulatory and quality strategy and submission authoring
- Drug/device assembly and packaging support.
The Insight by Nemera development and consulting organisation can support customers at any stage of development as a fully integrated or standalone partner. With locations in the US and Europe, Insight brings experience with a wealth of device types, patient populations and regulatory pathways. This broad experience and capabilities allows it to act as a partner and extension of customer teams delivering best-in-class solutions, ensuring no compromises are made when collaborating.
Willing to provide a fully automated industrial line to its partners, Nemera has invested in a new plant, offering its capability to produce prototypes and small series for clinical batches as well as large-scale automated volumes. Gathering state-of-the-art equipment from moulding to assembly and quality control testing, this new facility (Figure 5) includes an ISO 8 clean room and complies with the Building Research Establishment Environmental Assessment Method (BREEAM) recommendations. For instance, heat is recovered from the process line, and the facility also segregates and sorts all waste, aiming at recycling 100%.

Figure 5: State-of-the-art manufacturing facility, hosting cleanrooms compliant with BREEAM recommendations.
BENEFITS OF PARTNERING WITH AN INTEGRATED PRODUCT AND SERVICE PARTNER
Successful navigation of challenges often requires a partner with a broad set of capabilities and services. Nemera’s integrated device portfolio, development, consulting and manufacturing services allow customers to achieve the outcome of a successful regulatory submission and commercial launch of safe, effective and differentiated combination products, with a single partner applying an agile process across the device and combination product value chain. Ultimately, the benefits of this approach will drive:
Patient Centricy and Engagement
The needs of patients and clinical stakeholders are at the centre from the start of the programme to develop a custom patient engagement strategy that is consistently considered throughout the process to maximise effectiveness when launched into the market to drive loyalty from stakeholders.
Navigating the Combination Product Ecosystem
Nemera understands that developers must navigate a complex ecosystem to achieve market success. Its services are aligned to help clients meet the expectations of key stakeholders, including healthcare professionals, networks, payers and regulators to ensure that their needs are met throughout the process.
Flexibility to Focus on Your Core
The value of Nemera’s integrated services lies in the flexibility they offer, allowing customers to focus on their core business of drug discovery and development. Nemera offers best-in-class solutions for devices and combination products, ensuring that no compromises are made when collaborating with the organisation.
Reduction of Risk and Increased Speed of Market Access
A single partner working across the journey limits transitions between suppliers and ensures consistent execution of strategy. Nemera also provides a wealth of options for developing and realising a device to accelerate time to registration and market from small-batch to large-scale manufacturing and any interim supply required in between. When combined with the company’s service offering, this can significantly reduce timelines.
Looking to the future in this area, Nemera is just at the beginning of the journey because several pathologies are currently being studied to be treated with the same drug type. Some of them include obesity comorbidities in the cardiovascular field, which undoubtedly means treating more patients and extending the age range from paediatrics to elderly patients.
It goes without saying that sustainability is a priority in every aspect of Nemera’s daily work. The new facility is a good example but the company is also engaged in working towards eco-materials and eco-design, as well as energy sourcing and consumption, and has committed to several targets set by standard organisations such as the Science Based Targets initiative.5
REFERENCES
- “Obesity and overweight”. WHO, Mar 1, 2024.
- “One in eight people are now living with obesity”. Press Release, WHO, Mar 1, 2024.
- “WHO acceleration plan to stop obesity”. WHO Publication, Jul 3, 2023.
- NCD-RisC, “Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults”. The Lancet, 2024, Vol 403 (10431), pp 1027–1050.
- “Ambitious corporate climate action”. Web Page, Science Based Targets, accessed May 2024.

