To Issue 151
Citation: McBride DiLauri B, “BD Innovation for Subcutaneous Delivery of Large-Volume Complex Biologics.” ONdrugDelivery, Issue 151 (Sep 2023), pp 22–24.
Beth McBride DiLauri summarises the results of a clinical study on the BD Libertas™ Wearable Injector. The results position the device as a viable alternative to traditional intravenous administration.
From human factors engineers in the medical affairs organisation to line operators in the company’s manufacturing facilities, everyone at BD recognises that there is a patient at the centre of everything they do.
The BD Medical – Pharmaceutical Systems business regularly conducts a combination of primary market research, human factors studies and clinical research studies to understand the patient experience better. These insights are applied to injection device design and combination product development.
BD is focused on high-growth therapy areas, such as obesity and diabetes, as well as other leading chronic diseases. The company aims to apply its organisational insights into the patient experience, together with its injection expertise, to support treatment of chronic diseases and transition to new care settings while positively impacting patients.
“The study demonstrated consistent device functionality and acceptability across the study population tested.”
BD recognises that the patient experience can impact adherence and treatment outcomes, as highlighted by the US Association for Healthcare Research and Quality (AHRQ) and other leading organisations.1,2 The BD Libertas Wearable Injector programme is an example of BD’s work to enable the transition of care to new settings by shifting from intravenous (IV) to subcutaneous (SC) delivery.
To de-risk that transition, BD conducted interviews with patients and pharma companies, as well as multiple generative and formative human factors studies in an iterative cycle, to inform usability and the patient experience with a wearable combination product. The company also conducted its own clinical study* of the BD Libertas Wearable Injector, the results of which were published in Clinical Translational Science in 2021.3
This study assessed key parameters, including subject tolerability and device acceptability, which can impact the patient experience. Among the findings was confirmation that 100% of subjects were likely to use the BD Libertas Wearable Injector, if prescribed.3 This suggests a positive patient experience, leading to improved compliance.
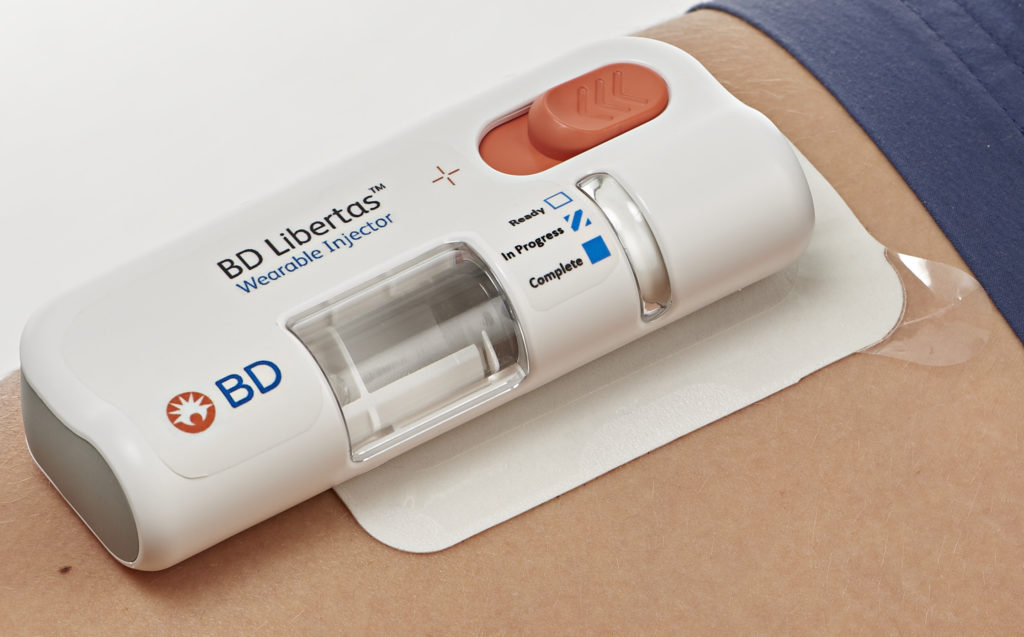
Figure 1: The BD Libertas™ Wearable Injector is designed to deliver SC injections of large-volume and/or high-viscosity fixed-dose complex biologics.
STUDY RESULTS IN DETAIL
The BD Libertas Wearable Injector (Figure 1) is designed to deliver SC injections of large-volume (2–5 mL or 5–10 mL) and/or high-viscosity (up to 50 cP) fixed-dose complex biologics in home or clinical settings. The 2–5 mL device (Figure 2) was evaluated in an early feasibility clinical study, using a viscous placebo, for its functional performance, tissue effects, pain tolerability and overall acceptability among 52 healthy participants.
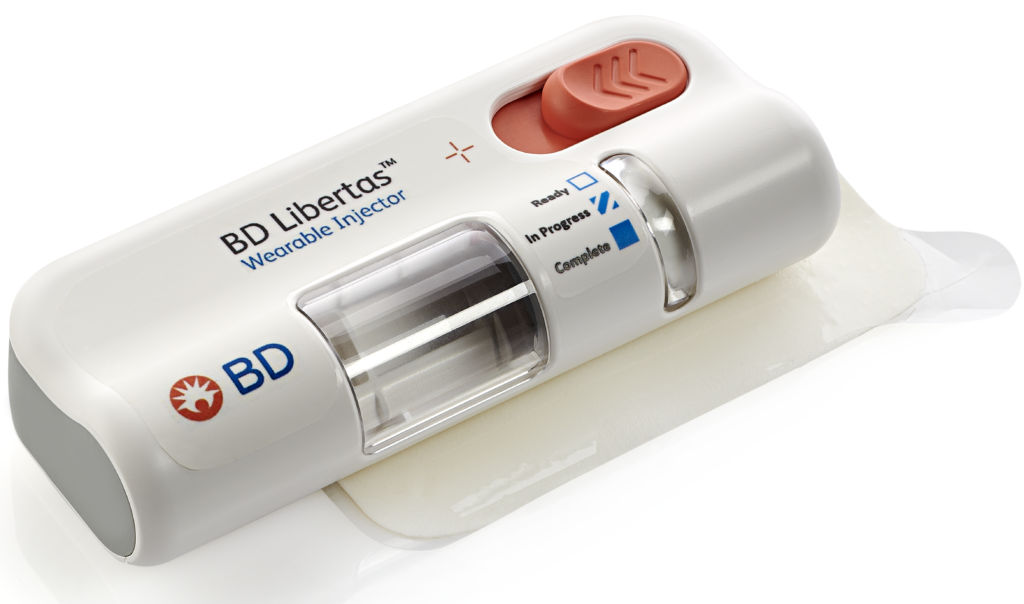
Figure 2: The 2–5 mL BD Libertas™ Wearable Injector.
The study demonstrated consistent device functionality and acceptability across the study population tested. Reliable targeted delivery was demonstrated by the fact that 98.6% of injections were successfully delivered within the targeted SC tissue – 93.2% completely SC and 5.4% predominantly SC. When it came to tolerability, the transient injection pain was well tolerated, with rapid resolution. On the safety front, no serious adverse events were reported.
Consistent and reliable functional performance was demonstrated by the study, confirming that 5 mL (±5%) of the placebo bolus was delivered to the SC tissue. Consistent performance was demonstrated across injection sites, gender, age and body mass index (BMI) categories tested, with and without movement during injection. Three BMI categories were tested – normal, overweight and obese – and two age groups, 18–64 years and ≥65 years. There was consistent injection delivery and duration – with a mean of 5.42 minutes. Reliable adhesive and needle actuation/retraction performance was also documented.
Any pain and tissue effects reported during the study were transient, with rapid resolution and broad acceptability. The results showed that ≥94% of participants found the transient pain acceptable at the end of the injection, with 96% reporting the injection site appearance acceptable after receiving all four injections. In addition, ≥92% said the device was comfortable to wear during injection and ≥98% found the adhesive removal acceptable. When it came to the abdomen and thigh injection sites, the 52 study participants found both sites equally acceptable overall – with 25% preferring the abdomen, 26.9% preferring the thigh and 46.2% having no preference (Figure 3).
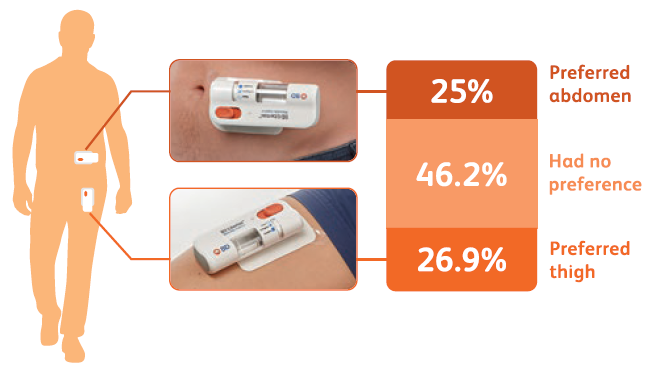
Figure 3: The 52 subjects found both injection sites equally acceptable.
“BD is committed to evolving its capabilities and portfolio to meet pharma companies’ requirements, including supply chain stability, partner agility and sustainability.”
KEY BENEFITS
The key benefits of the BD Libertas Wearable Injector include the fact that the patient does not have to do any filling or assembly of the device. There is also automatic needle insertion and retraction, and the device is fully mechanical – with no software or electronics components.
Compared with the IV route, SC administration offers3–5:
- Increased patient autonomy and convenience
- Reduced treatment cost, time and systemic effects
- Self or caregiver administration outside hospitals in new care settings, such as at home.
Added to that, wearable injectors can be capable of delivering wider volume and/or viscosity ranges than other delivery system options currently on the market. The BD Libertas Wearable Injector’s range of 2–5 mL or 5–10 mL and up to 50 cP is an example of this.
BROAD PORTFOLIO
In addition to the patient experience, BD also recognises that there are multiple key decision criteria that inform pharma companies’ choices when selecting the optimal device for a given combination product. BD is committed to evolving its capabilities and portfolio to meet pharma companies’ requirements, including supply chain stability, partner agility and sustainability.
BD provides a broad portfolio of parenteral drug delivery systems, including glass and plastic prefillable syringes, safety and shielding systems and advanced drug delivery systems, including pens, autoinjectors and wearable and on-body injectors. The company also offers a wide range of combination product development testing services, leveraging its deep expertise partnering in combination product development.
BD Libertas™ Wearable Injector is a product in development; some statements are forward looking and subject to a variety of risks and uncertainties. BD Libertas Wearable Injector is a device component intended for drug-device combination products and not subject to FDA 510(k) clearance or separate EU CE marked certification.
*Early feasibility clinical study of investigational BD Libertas™ Wearable Injector evaluated 5 mL, non-Newtonian ~8cP SC placebo injections in 52 healthy adult subjects of ≥18.5 kg /m2 BMI divided into two age groups (18–64 or ≥65 years) for functionality, tissue effects, subject tolerability and acceptability.
Find out more about BD Libertas Wearable Injector at: drugdeliverysystems.bd.com/products/self-injection-systems/libertas-wearable-injector.
REFERENCES
- “The Consumer Assessment of Healthcare Providers and Systems (CAHPS) Ambulatory Care Improvement Guide: Practical Strategies for Improving Patient Experience”. AHRQ, May 2017, pp 2–3.
- Manary MP et al, “The Patient Experience and Health Outcomes”. NEJM, 2013, Vol 368, pp 201–203.
- Woodley WD et al, “Clinical Evaluation of an Investigational 5 mL Wearable Injector in Healthy Human Subjects”. Clin Transl Sci, 2021, Vol 14(3), pp 859–869.
- Woodley WD et al, “Clinical Evaluation of an Investigational 5 mL Wearable Injector in Healthy Human Subjects”. Clin Transl Sci, 2021, Vol 14(3), pp 859–869.
- Collins D, Sanchez-Felix M, Badkar A, Mrsny R, “Accelerating the development of novel technologies and tools for the subcutaneous delivery of biotherapeutics”. J Control Release, 2020, Vol 321, pp 475–482.
- Bittner B, Richter W, Schmidt J, “Subcutaneous administration of biotherapeutics: an overview of current challenges and opportunities”. BioDrugs, 2018, Vol 32(5), pp 425–440.

