To Issue 148
Citation: Ecenarro Probst S, “Challenges and Achievements of TiO2 Replacement in Hard Capsules.” ONdrugDelivery, Issue 148 (May/Jun 2023), pp 22–26.
Susana Ecenarro Probst looks at the issues arising in replacing TiO2 in hard capsules and introduces Qualicaps‘ alternative hard capsule formulation.
Titanium dioxide (TiO2) – also known as E-171 – is widely used in the pharmaceutical industry for solid oral dosage forms, such as tablets, hard capsules and soft capsules, as an opacifier and white colourant. The worldwide use of this excipient and food additive began to be threatened due to some concerns raised by the French consumer healthcare community in 2018, which drew special attention to the so-called nanomaterial risk that this ingredient could have inherently present. In April 2019, an official communication from the French authorities finally announced the prohibition of TiO2 as a food additive in the first country in Europe. The ban started on January 1, 2020, and the first one-year period was extended until December 2021.
“The EMA published a Q&A paper on relevant technical aspects related to the pharmaceutical quality of TiO2 replacement in medicinal products, encouraging the pharmaceutical industry
to make all possible efforts to speed up the investigation of new alternatives for replacing E-171 in drug products.”
Nevertheless, by 2015, the European Commission had already requested the European Food Safety Authority (EFSA) to review the safety of E-171 as a food additive, to which an EFSA panel concluded in June 2016 that no real concerns existed about the continued safe use of E-171. Despite this, the EFSA recommended that more testing should be performed to complete the toxicological studies.
Finally, in May 2020 EFSA published an updated scientific opinion regarding E-171 toxicity after assessing the latest toxicological information and studies available.1 It concluded that, based on all currently available evidence, genotoxicity concerns could not be ruled out and the E-171 TiO2 food additive could no longer be considered safe.
Because TiO2 is extensively used in medicinal products as a colourant and opacifying agent (mainly tablet film coating and hard-shell capsule formulation), Commission Regulation (EU) 2022/63 announced in January 2022 that, for the time being, TiO2 remained on the list of authorised additives to allow its use in medicinal products to avoid any shortage of medicinal products that may impact public and animal health. A review clause stated that, after three years, an updated assessment would be issued by the EMA and provided to the European Commission by April 2024 for further decisions.
The EMA published a Q&A paper on relevant technical aspects related to the pharmaceutical quality of TiO2 replacement in medicinal products, encouraging the pharmaceutical industry to make all possible efforts to speed up the investigation of new alternatives for replacing E-171 in drug products.
In response to questions from the QWP Expert/EMA, an interesting survey conducted by the three European associations representing human medicines manufacturers (AESGP, EFPIA and Medicines for Europe) revealed that out of all the marketing authorisations for products containing TiO2 in European Economic Area (EEA) countries (June 2021), the EV Codes (unique codes assigned to any entity (e.g. substance, product, etc.)) representing oral pharmaceutical forms containing TiO2, accounted for 63% of tablets and 95% of capsules.2
Derived from the last EMA published document, the following relevant considerations and concerns have been raised and discussed for marketed medicinal products by the pharmaceutical industry in different conferences, held in Europe during the last year:
- A review of the risks related to business, regulatory and technical matters needs to be performed by pharma industries as many products are impacted.
- The EMA’s strong recommendation communicated to the pharma industry to accelerate the research and development of alternatives is crucial to evaluate the feasibility of the replacement and be able to present a consolidated review in April 2024 based on “objective verifiable” scientific information.
- Detailed safety and quality technical information, including stability data, should be provided in a short timeframe in the case of tablets and capsule suppliers for the complete evaluation of new alternative candidates, to initiate the manufacturing of medicinal products and pilot stability studies, where appropriate.
- The new tablet and capsule alternatives should have no manufacturing capacity constraints and become commercial at a reasonable cost.
- The possible 1–1 replacement of calcium carbonate for TiO2 may not be an adequate solution as studies have shown that the final appearance did not achieve the same colour result in several marketed products.
- The handling and decision-making position on new variation submissions of existing worldwide marketed medicinal products could become country- or region-dependent due to a possible discrepancy between a potential suspension of TiO2 by the European Commission in 2025 (decision based on the EMA review) and some important countries, such as the US, Canada and the UK, which have already expressed and published their arguments against the prohibition of this excipient.
- The EMA Regulatory Authority would have to be able to assume all these new dossier variations submissions and be able to respond within an acceptable timeframe to the pharma companies.
“Hard capsule manufacturers have undergone some important challenges in investigating and developing a new replacement alternative expected to reproduce or emulate the TiO2 properties.”
Over the last few years, hard capsule manufacturers have undergone some important challenges in investigating and developing a new alternative expected to reproduce or emulate the TiO2 properties of what has been considered for decades as a well-established white pigment, opacifier and protector of UV light. The following action items have been, therefore, thoroughly revised:
- Assessment of the regulatory framework (per International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use regions) for the new TiO2 alternative and subsequent viable hard capsule drug product dossier variation submissions.
- A review of safety risks as heavy metals and the content of nanomaterial, the latter associated with potential toxicity as indicated by the EFSA; the determination of the particle size distribution and subsequently the assurance of nanoparticles content absence (1–100 nm) or the compliance with the content percentage stated by the current and latest recommendation of a nanomaterial definition as per the European Commission (Commission Recommendation of 10.6.2022 on the definition of Nanomaterial):
“‘Nanomaterial’ means a natural, incidental, or manufactured material consisting of solid particles that are present, either on their own or as identifiable constituent particles in aggregates or agglomerates, and where 50% or more of these particles in the number-based size distribution fulfil at least one of the following conditions:
(a) one or more external dimensions of the particle are in size range of 1 nm to 100 nm.
(b) the particle has an elongated shape, such as a rod, fibre or tube, where two external dimensions are smaller than 1 nm, and the other dimension is larger than 100 nm.
(c) the particle has a plate-like shape, where one external dimension is smaller than 1 nm, and the other dimensions are larger than 100 nm.” - The quality verification referred to:
– Opacity (contrast index) and transmittance (UV protection) suitable results for an acceptable appearance of a white and coloured opaque capsule that will represent stable UV protection for photosensitive formulation ingredients.
– Critical quality attributes within the specification of stability study capsule batches (disintegration, dissolution test, brittleness, weight and dimensional parameter). - The control of the colourant replacement effectiveness related to the TiO2 functional properties as a colourant, opacifier, UV protector and not generally reactive substance implies:
– The achievement of colour suitability for existing marketed drug products (minimising the patient impact due to appearance changes) for new hard capsule developments, with an adequate match to a Pantone colour reference.
– The selection of not generally reactive alternative component(s) within the hard capsule composition.
– Good mechanical properties, including:
. Absence of brittleness or the same performance as with TiO2-containing hard capsules.
. Surface smoothness with results according to TiO2 capsule shells.
. Machinability/runnability with several filling machines at different speeds.
– Capsule shell UV laser imprinting/ marking suitability.
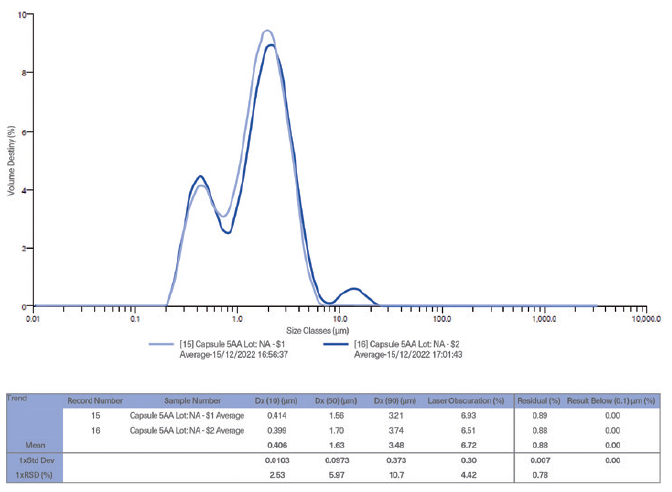
Figure 1: PSD for HPMC capsule. Note: Analytical Instrument: Mastersizer 3000 apparatus (Laser Diffraction).
As mentioned, one of the critical challenges has been the achievement of an acceptable colour opacity and whiteness and the certainty to match different existing colours by changing the opacifying agent amount depending on the desired final colour. Calcium carbonate, as a unique direct substitution for titanium dioxide, cannot fully achieve this purpose for all product colour requirements of pharma companies.
“The estimated proportion of medications including TiO2 as an excipient is vast.”
Qualicaps Europe has been developing a new hard capsule formulation by carefully evaluating different opacifying agents in recent years. After conducting a comprehensive evaluation of existing available excipients regarding safety, regulatory and quality considerations, the company decided to focus on the most suitable options based on the previously mentioned aspects. The incorporation of selected new opacifying agents, together with a consistent and robust manufacturing process, have been successfully validated for hydroxypropyl methylcellulose (HPMC) and gelatin TiO2-free hard capsules.
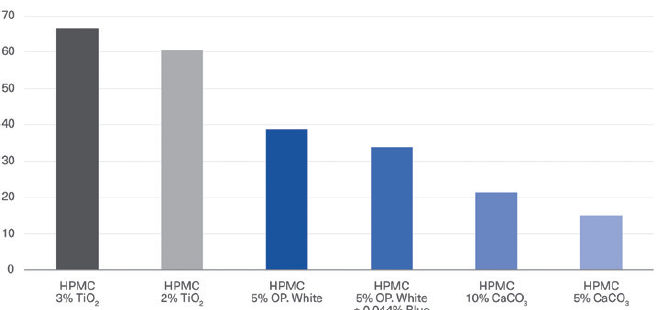
Figure 2: Comparison of Quali-V® containing TiO2 versus different Quali-V® TiO2-free capsules with different opacifying agents in the composition.
Some relevant scientific evidence data, compiled under a capsule product technical dossier, are presented here as an example:
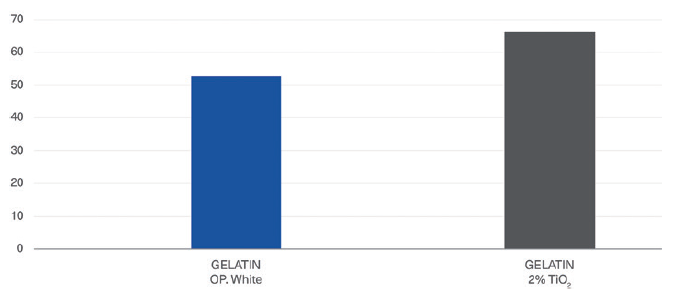
Figure 3: Comparison of Quali-G™ containing 2% TiO2 versus Quali-G™ TiO2-free capsule with opacifying agent in the composition (Gelatin OP. White).
- Safety-standard-related results for empty capsule evaluation: particle size distribution (PSD) for HPMC capsule (Figure 1). The final capsule product testing results with the new opacifying agent have shown an absence of nanoparticles.
- Quality-standard-related results for empty capsule evaluation:
a. Opacity results: The opacity or contrast index for Quali-V® with TiO2 was compared with different Quali-V® TiO2-free capsules with different opacifying agents in the composition (Figure 2). The opacity for Quali-G™ TiO2-free with an opacifying agent was compared with Quali-G™ containing 2% TiO2 (Figure 3).
b. Transmittance results: Transmittance results are presented in Figures 4 (HPMC capsules) and 5 (gelatin capsules), comparing different colour formulations.
c. Disintegration and dissolution results: The empty Quali-V® TiO2-free capsules comply with a final disintegration time acceptance criteria of NMT 15 minutes tested as per the Ph Eur Test (chapter 2.9.1. “Disintegration of tablet and capsule” of European Pharmacopoeia 11th Edition). The dissolution profiles of TiO2-free capsules are like standard Quali-V® TiO2 capsules complying with EP Ed 11.0th monograph, 2.9. - “Dissolution Testing for oral dosage forms monograph” and Chapter 5.17.1 “Recommendations on Dissolution Testing for immediate release-dosage forms”, in which the acceptance criteria is stated as no less than 80% of dissolved API amount in less than 45 minutes. Figures 6 and 7 show the results of three batches without TiO2 versus one with TiO2 as a reference analysed with buffer solutions at pH 1.2 and pH 6.8.
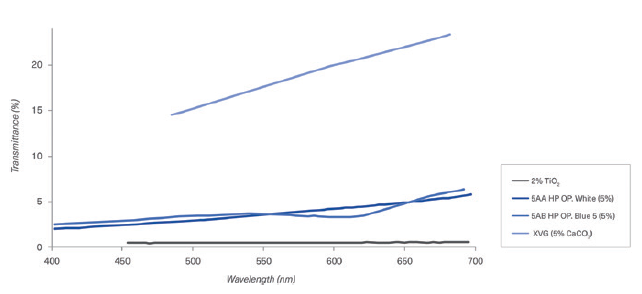
Figure 4: Comparison of different colour formulations of HPMC capsules containing TiO2, CaCO3 and opacifying agent in the composition.
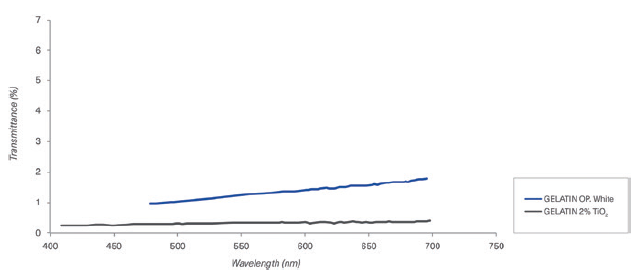
Figure 5: Comparison of different colour formulations of gelatin capsules containing TiO2 and opacifying agent in the composition.
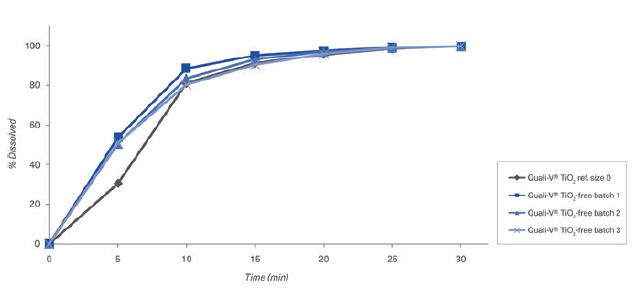
Figure 6: Dissolution profiles of three different batches of Quali-V® containing TiO2 and opacifying agent with buffer solutions at pH 1.2 (Capsule fill formulation: acetaminophen 20% and lactose 80%. Dissolution test method: paddle at 50 rpm).
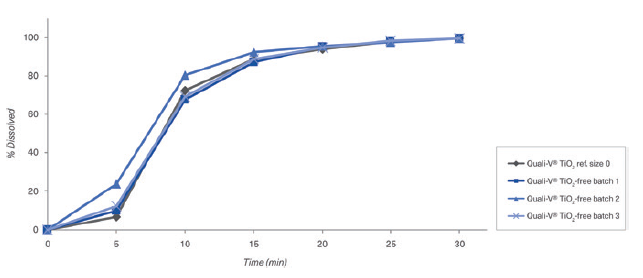
Figure 7: Dissolution profiles of three different batches of Quali-V® without TiO2 and one of Quali-V® containing TiO2 with buffer solutions at pH 6.8 (Capsule fill formulation: acetaminophen 20% and lactose 80%. Dissolution test method: paddle at 50 rpm).
FINAL REMARKS
Since the European Commission announced a potential ban on using TiO2 as an excipient, the pharma industry has been actively searching for a replacement for this component extensively used in different oral dosage forms for marketed human medicinal products. The estimated proportion of medications including TiO2 as an excipient is vast, representing – in the case of ingested pharmaceutical capsules – more than 90% of the total number of marketing authorisations containing TiO2 in EEA countries (April 2021).
The efforts behind finding an alternative for this well-established excipient as an opacifier, whitening colourant and other functional properties have been a priority in the last few years for hard capsule manufacturers. Qualicaps has developed a suitable alternative with TiO2-free HPMC and gelatin hard capsules fulfilling the expected safety, quality and effectiveness standards required, and providing excellent appearance and performance compared with existing TiO2-containing capsules.
REFERENCES
- “Safety assessment of titanium dioxide (E171) as a food additive”. EFSA, May, 2021.
- “Use of titanium dioxide as excipient in human medicines”. Feedback submitted to EMA on July 2, 2021.

