To Issue 149
Citation: Williams C, Weber N, Fuensalida Pantig G R, “Collaborative Innovation in Connected Autoinjectors: Creating High-Impact Adherence Solutions.” ONdrugDelivery, Issue 149 (Jun 2023), pp 48–54.
Chelsea Williams, Nils Weber and Gene Rhode Fuensalida Pantig discuss the potential of collaborative innovation in connected drug delivery when it comes to creating high-impact, low-touch adherence solutions.
Stakeholder groups – including patients, healthcare providers and payers – all have unique demands and expectations. Patients seek greater convenience and improved quality of life when choosing a treatment modality. For healthcare providers, there is a deep desire to deliver higher quality and more personalised care that requires fewer resources. And for payers, it is critical that adherence levels improve patient outcomes and, in turn, mitigate the lifetime cost of care.
“The industry is at a crossroads to implement and scale connected solutions that harness the power of digital health.”
As we continue to witness an irreversible shift in the development of home-based medicines, there is also an increased demand for augmented devices that can monitor the efficacy of such home therapies. With a dramatically increasing number of drugs becoming available in autoinjectors for self-injections, it raises questions about how pharma and medtech can forge better partnerships to design connected therapeutics that aid in treatment adherence and improve patient outcomes. Equally important, such digitalisation should gravitate towards empowering the patient in treatment-related decisions.
DIGITAL HEALTH AND THE EVOLUTION INTO CONNECTED THERAPEUTICS
More than 30 years ago, the US FDA approved the EpiPen (Mylan, part of Viatris, PA, US) for the self-administration of adrenaline (epinephrine) to treat allergic anaphylactic reactions and, since then, autoinjection devices continue to improve the lives of millions of patients across the world. The popularity of autoinjectors for home use surged in the mid-2000s, driven by a dramatic increase in the number of frequently dosed biologics and the patient drive towards at-home care. Notable launches included AbbVie’s Humira (adalimumab) and Immunex/Amgen’s Enbrel (etanercept), both in 2006.1
Much of the success of autoinjectors comes down to a combination of safety, simplicity and convenience, which empowers patients and caregivers to self-administer essential therapies at their chosen time and location, reducing the burden on healthcare clinics and providers. Particularly for those suffering from chronic conditions, these qualities can avoid the need to continually attend healthcare clinics for treatment, liberating their time while also reducing the cost for payers and the burden on the healthcare professionals (HCPs) tasked with optimising the management of their patients’ healthcare. Across the world, these factors have helped propel home-based self care to new heights in recent years.
While the pandemic forced a detachment between patients and HCPs, it could be said that it also accelerated the development and implementation of technologies that allow for remote care. For drug delivery devices, the use of connectivity is still being explored to uncover its potential. Digital health is complex and there is no one-size-fits-all approach to generating value from connecting and digitalising treatment modalities. However, the stride to health innovation will continue to drive the five Ps in healthcare (i.e. providers, payers, policymakers, pharma and the patient) to better understand the added value digitalisation brings to at-home treatments – and data will be key to this realisation.
With new developments in the space – e.g. more remote clinical visits, the introduction of digital therapeutics (DTx) and prescription DTx (PDT), and regulatory guidance on the implementation of decentralised clinical trials – the industry is at a crossroads to implement and scale connected solutions that harness the power of digital health.2–4
Transforming Concepts into Reality Through an Innovation Partnership Framework
To close the gap between conceptual testing and unleashing the real-world value of connected therapeutics, SHL Medical developed the Innovation Partnership Framework. This framework allows SHL and its partners to co-develop connected solutions and evaluate different use cases on a smaller scale through an agile approach (Figure 1). Key to realising this ambition is establishing a collaborative partnership with pharmaceutical and biotechnology partners while fostering knowledge sharing and challenging the status quo. For combination products, such an integrated process ensures that all aspects of a drug-device are considered in parallel, and that the resulting solution creates value that is relevant across the five Ps in healthcare.
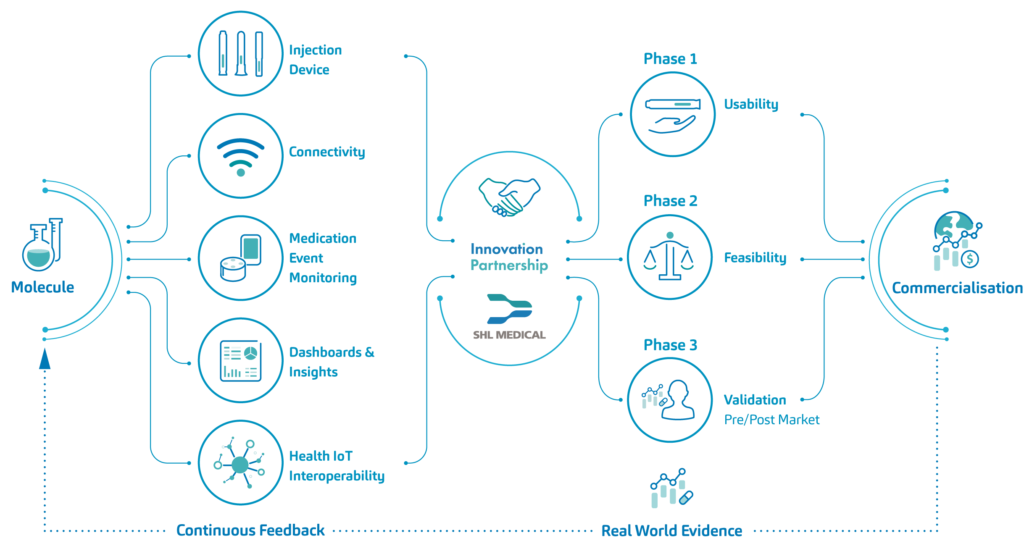
Figure 1: An overview of SHL Medical’s Innovation Partnership.
“All elements were designed to provide patients with an autonomous platform for managing self-injections in line with their therapy regimen.”
Development of the Accompanying Digital and Connected Technology
An important element of SHL Medical’s Innovation Partnership is the technology, and through SHL’s application expertise, various emerging technologies have been in constant development in recent years. As early as 2016, SHL Medical presented an early connected autoinjector concept – a device featuring a recording unit that logs and transmits injection data.
Also, within the last decade, SHL Medical explored other technological initiatives and has been Innovation Zed’s official partner for connected pen injector solutions. Through active collaboration, SHL provided its technical expertise to Innovation Zed in the design and development of the InsulCheck family of devices – a range of connected add-on products that support the monitoring of disease management regimens.
One of the learnings from SHL’s first connected autoinjector concept was that a suitable connected solution must come with the lowest possible impact on the device, through a so-called low-fidelity product refined through an incremental innovation model. This means that the solution should safeguard ease of use with no extra user steps, circumvent potential regulatory roadblocks for pharma and ensure manufacturability, as well as implement the principles of design for sustainability.
In this context, a clear opportunity for connectivity was identified, building upon the simplicity of SHL’s market-proven Molly® modular platform. In particular, the addition of sensing technology and wireless communication would allow a digitally enhanced autoinjector platform to generate and transmit data on patient use and to support improved adherence to therapy regimens without further oversight from HCPs or the patient making additional clinic visits. The ensuing process gave rise to the Molly Connected Cap (Figure 2).
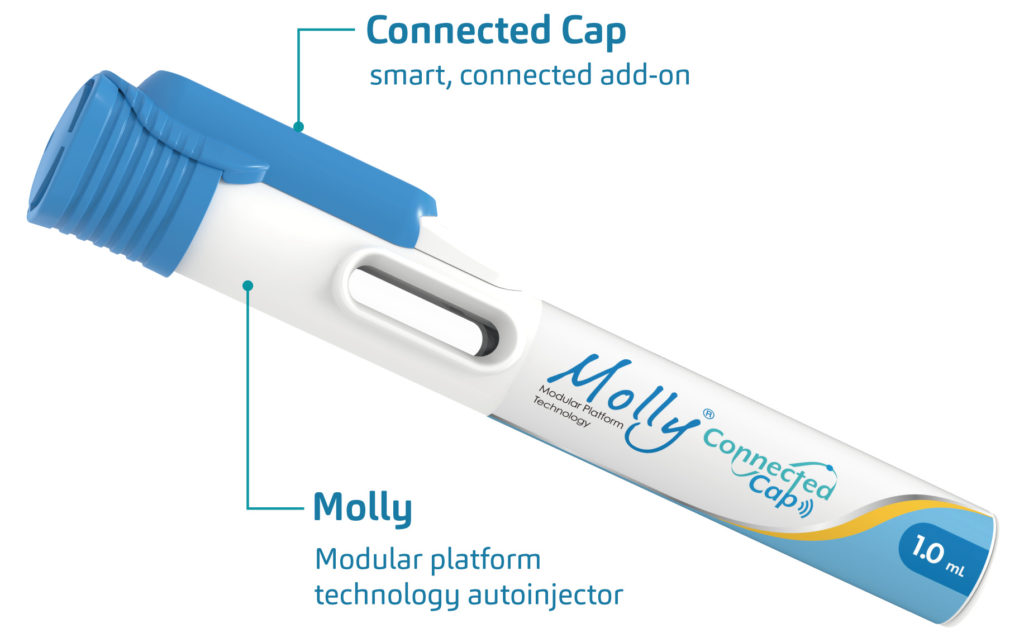
Figure 2: An overview of the Molly Connected Cap – a compact, retrofittable autoinjector add-on that records and transmits data on patients’ use of the device.
The Molly Connected Cap: Complex Problems Require Simple Solutions
The Molly Connected Cap is a compact, retrofittable autoinjector add-on that records and transmits data on patients’ use of the device. Upon cap removal from the Molly device, the Connected Cap becomes active, allowing timestamped data to be relayed through a smart data transmission hub and to the cloud, which provides audio and visual cues to a patient on the timing of forthcoming injections. Accompanying demonstration software, accessed via either a mobile app or web browser, was also developed to further provide patients with an assistive interface to their Molly autoinjector, providing patients with injection reminders, injection history and scheduled injection information.
SHL’s Innovation Partnership and Molly Connected Cap in Action
With a strong use case for a connected autoinjector to benefit patients with chronic conditions, SHL Medical established an Innovation Partnership with one of its longstanding pharmaceutical partners, aimed at retrofitting connectivity to a version of its Molly-based autoinjector product in a different injection modality, complete with the associated data-management system and patient software interface. Taken together, all elements were designed to provide patients with an autonomous platform for managing self-injections in line with their therapy regimen.
Having completed the transition from early feasibility to proof of concept, a series of assessments was carried out to validate whether the Connected Cap would, in practice, deliver a user experience to the benefit of patients suffering from chronic neurological, as well as autoimmune and inflammatory, conditions.
A formative usability study, led by SHL Medical in close collaboration with its pharma partner, assessed users’ overall interactions with the Connected Cap, with a particular focus on preference, safety and effectiveness. The study also evaluated the concept and design of the entire Connected Cap system for whether the injection reminder, injection recording and injection process satisfied users’ needs.5
A Look into Real-World Evidence
In one of the studies explored with an Innovation Partner, 31 patients from a mixed age range, gender and disease severity – among other demographics – were recruited in the US Midwest, all of whom had experience with self-administering injections to manage a diagnosis of chronic neurological, as well as autoimmune and inflammatory, conditions. They were asked to perform multiple simulated injections in each of three separate scenarios: only using a Molly autoinjector (use scenario A); Molly + Connected Cap + mobile app platform (use scenario B); and Molly + Connected Cap + smart data transmission hub (use scenario C). Scenarios were counterbalanced to minimise instruction bias.
Putting the study design and primary test materials into context requires an explanation of the Molly Connected Cap architecture. The Molly Connected Cap implements a wireless mode of connection powered by a Bluetooth Low Energy beacon. Its proprietary firmware is programmed to facilitate the seamless, encrypted transfer of data from the Connected Cap, either to a smartphone or a smart data transmission hub. From the smartphone or the smart data transmission hub, a cascade of data transmission is triggered – using Long-Term Evolution wireless broadband communication – with an encryption algorithm in place. In real scenarios, this means that the usual injection process is unchanged and, in the case of using the smart data transmission hub, connecting the module to a power source is the only extra step prior to injection.
Taken together, it could be said that the confluence of the many relevant factors might have affected the turnout of usability data and overall patient preference. For one, the use of the mobile app and smart data transmission hub were accessories integrated into the simulated injection process experience. In detail, scenario B required patient interaction with the Connected Cap and the mobile app, where it could be assumed that the general population is accustomed to operating smartphones and apps. For scenario C, patients interacted with an entirely new accessory, wherein the light indicator on the smart data transmission hub would flash to signify an injection reminder. This feedback lighting is coupled with a text message reminder triggered by the two-way communication feature (bidirectional data transmission) of the hub.
As an extra layer, a post-task interview was designed to elicit subjective data based on the patient’s experience of using the equipment. For the study, participants were provided with a set of prepared test materials to avoid any complications with software downloads or data transfer; they were also allowed to complete a training activity, which provided an opportunity to carry out a test injection using training equipment prior to the official simulation tasks. All the needle-based injection systems, in both the training and official tests, were activated on a pad; no self-injections were carried out as part of the project.
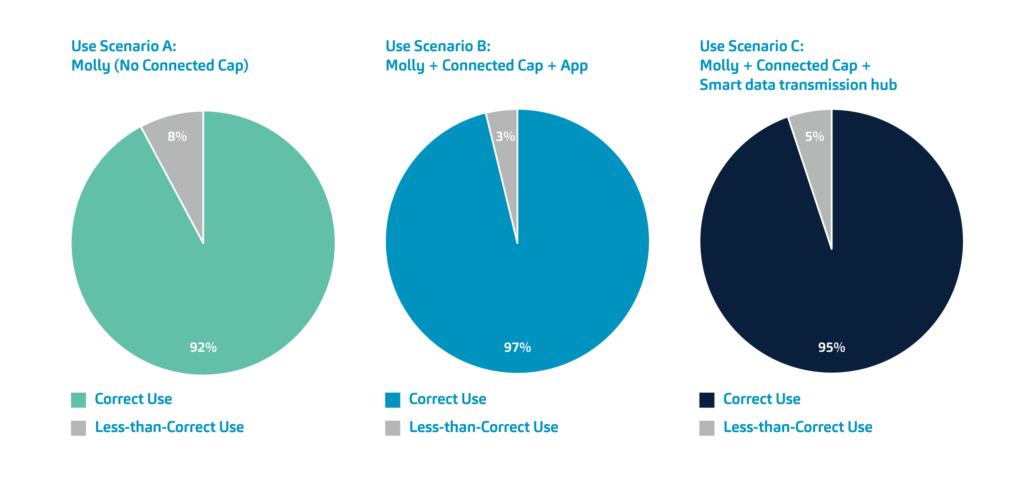
Figure 3: Summary of the formative usability study data. Users with chronic neurological, as well as autoimmune and inflammatory conditions were asked to simulate injections with the Molly autoinjector (use scenario A), Molly + Connected Cap + mobile app platform (use scenario B) and Molly + Connected Cap + smart data transmission hub (use scenario C).
Each simulated injection was evaluated objectively and graded according to specific criteria. To simplify the analysis(Figure 3), these criteria were grouped into either “correct use” or “less-than-correct use”, with the latter incorporating instances defined as “user error”. Across all three injection scenarios and all age ranges, participants used the equipment correctly in more than 92% of cases. This figure was highest (97%) for use scenario B. In total, recorded instances of less-than-correct use were 4.96% across all scenarios.
On the other hand, the subjective data (Figure 4) revealed no difference between concept-use scenarios A, B and C for ease of injection, with both Molly only (A) and all Molly + Connected Cap (B + C) scenarios registering 4.6 out of a possible 5. There was also a negligible difference recorded in the perceived force required to remove the device cap in each of these scenarios, with participants explaining that this action became easier over time as they became more familiar with the device. For many participants, this was their first interaction with a two-step autoinjector.
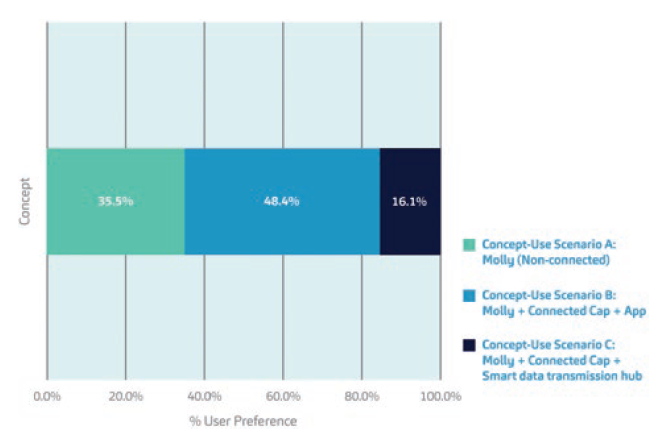
Figure 4: A readout of the overall preference rankings of the study participants for the concept-use scenarios employed in the formative usability study.
Overall, the Molly + Connected Cap + mobile app platform was the most preferred scenario (concept-use scenario B) among 48% of the study participants. Among the beneficial features cited were convenience, scheduling of reminders, automatic logging of injections and the ability to verify adherence. A total of 35% of the participants found the Molly-only scenario (concept-use scenario A) familiar and simple, while the Molly + Connected Cap + smart data transmission hub (concept-use scenario C) was the least preferred due to the requirement and potential burden of needing additional equipment.
The Impact of Harnessing the Power of Data
The formative study results affirm the idea that the Molly Connected Cap offers key distinctive advantages. For one, it provides patients with a standalone solution for managing their self-injections, reducing the need for frequent clinic visits and empowering patients to take control of their treatment. The visual and reminder cues provided by the smart hub and mobile app help patients adhere to their therapy regimen and ensure they receive their injections at the right time.
As a connected therapeutics platform (i.e. the Molly autoinjector, together with the Connected Cap, the smart data transmission hub, the app and the cloud), it enables healthcare providers – among other stakeholders – to monitor patient adherence remotely and intervene when necessary. Also, care providers and contract research organisations (CROs) have the opportunity to review patients’ injection histories, identify any deviations or missed doses, and provide timely support or adjustments to treatment plans. The platform improves the personalisation and efficiency of healthcare delivery, reduces the burden on HCPs, and produces a higher level of self-efficacy and engagement among patients.
The data collected by the Connected Cap opens up new possibilities for pharma and biotech companies to conduct real-world evidence studies and gather post-marketing insights. By analysing aggregated data on device use, medication use (e.g. Rx refills, drug shelf life and expiration), adherence rates, behavioural patterns and patient-reported outcomes, healthcare stakeholders can evaluate the effectiveness of therapies in real-world settings, identify areas for improvement, generate evidence to support reimbursement discussions with payors and identify potential hotspots for use patterns (e.g. looking into regions that suffer from minimal health literacy versus looking into health equity on a larger scale).
SHL’s most recent Innovation Partnership demonstrates the power of collaboration between pharma and medtech in the development of connected combination products. The flux of expertise and resources from both sides is closing the gap in care, in turn addressing the unmet needs of therapy self-management. Partnering early on can help accelerate the development and eventual commercialisation of connected therapeutics.
A PRESENT SOLUTION TOWARDS A BETTER-CONNECTED FUTURE
Technology such as the Connected Cap has real potential to address patient-related issues, tackling poor adherence and filling the information void with valuable data on medicine use. Importantly, the success of these studies also highlights how digital solutions do not necessarily require pharmaceutical companies to pursue risk-laden product developments from the ground up. Instead, the ability to digitalise existing, proven technologies – augmenting them through connectivity and complementary data-management systems – can open up new opportunities comparatively rapidly and with lower risk.
A layered approach to exploring the potentials of digital health – such as through the SHL Innovation Partnership Framework and the Molly Connected Cap – serves as a compelling example of how connectivity can be integrated into existing devices to provide a wealth of valuable data. A supporting device ecosystem (Figure 5) can ease the transition to connected therapeutics. Of equal importance, the results of SHL’s recent Innovation Partnership have also shown that collaborative frameworks can help generate meaningful data for pharmaceutical companies to better harness the power of digital health.
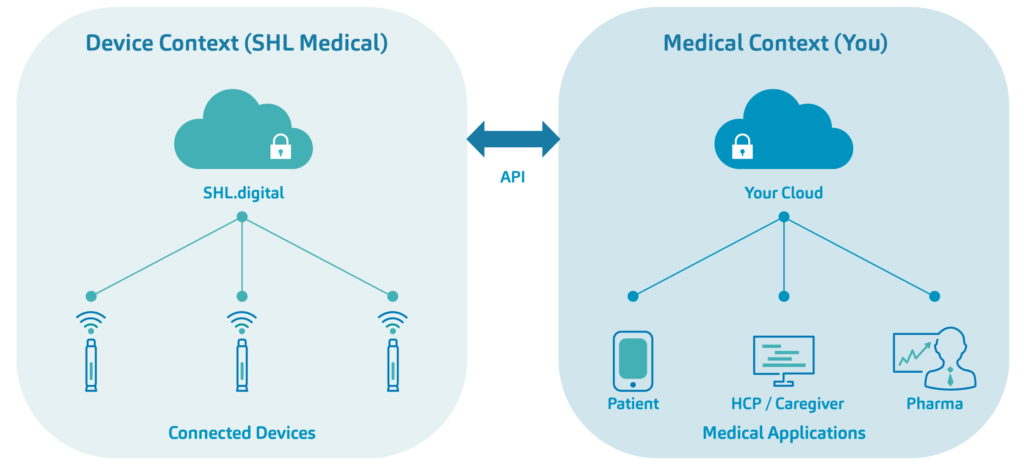
Figure 5: Device-driven solutions towards connected health – such as the Molly Connected Cap – facilitate the seamless integration of autoinjector combination products with an interoperable digital ecosystem.
SHL is in active partnerships with pharma, clinical, R&D, HCP and patient groups to identify what the value of connectivity means for each segment. With an Innovation Partnership Framework designed to facilitate various connected therapeutics programmes in parallel, SHL will continue to support the success of connected device piloting programmes and exploratory studies as a low-commitment, high-data-generation approach to power through digital health and connected therapeutics.
REFERENCES
- PharmaCircle, 2023.
- “Draft Guidance for Industry: Decentralized Clinical Trials for Drugs, Biological Products, and Devices”. US FDA, May 2023.
- “What is a DTx?”. Web Page, Digital Therapeutics Alliance, Accessed Jun 2023.
- Shafai G, Aungst TD, “Prescription digital therapeutics: A new frontier for pharmacists and the future of treatment”. J Am Pharmacists Assoc, Apr 2, 2003.
- Formative Usability Study, SHL Medical, 2023 (unpublished).

