To Issue 168
Citation: Katz M, Ratigan M, “Empowering Pharma with Innovative Infusion Technologies Amid US Policy Changes”. ONdrugDelivery, Issue 168 (Jan 2025), pp 124–127.
Mindy Katz and Michael Ratigan outline the implications for pharmaceutical manufacturers of recent US policy changes – and highlight how Eitan Medical’s portfolio of infusion solutions can offer support.
The dynamic intersection of regulatory policy and pharmaceutical innovation has once again taken centre stage as the US undergoes administration changes, while simultaneously working through the evolution and effects of the Inflation Reduction Act (IRA) and the Recovering Excessive Funds for Unused and Needless Drugs (REFUND) Act. Understanding the implications of these Acts for pharmaceutical manufacturers (Figure 1), both in the US and abroad, demands rigorous examination, while embracing a level of uncertainty in light of the presidential administration transition.
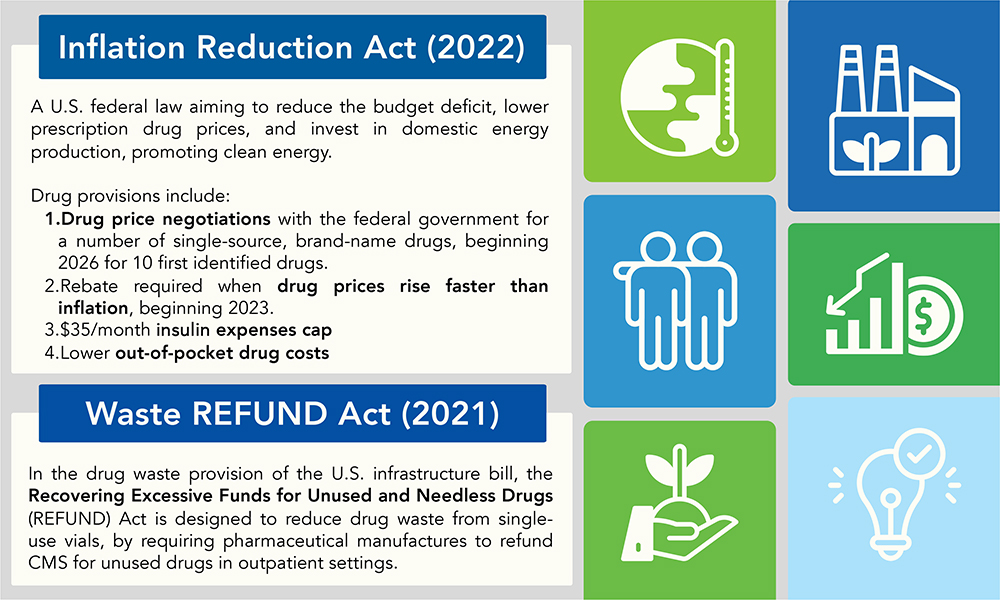
Figure 1: US legislation implications for pharmaceutical manufacturers.
This evolving landscape underscores the potential for drug delivery partnerships to successfully address the complexities of modern healthcare trends. Eitan Medical’s expertise in infusion technology, together with its commercial product experience, global presence, flexibility in pharmaceutical partnerships and robust portfolio of connected, infusion solutions – including the Sapphire™ and Avoset™ infusion pump platforms and the Eitan Insights™ digital health platform – position it as a pivotal partner of the pharmaceutical industry as it navigates these challenges.
LEGISLATIVE CONTEXT: IRA AND REFUND ACT
The IRA, signed into law in 2022, fundamentally shifts the dynamics of drug pricing policies in the US market. Key provisions include the ability for Medicare to negotiate prices for several recently identified high-cost drugs, penalties for price increases that outpace inflation, and caps on insulin supplies and out-of-pocket expenses for beneficiaries. While this aims to promote affordability and patient access across the nation, it also introduces challenges for pharmaceutical manufacturers, as expensive drugs and biologics eligible for direct price negotiations with Medicare may experience significant price cuts, unplanned for at this stage of their lifecycle.
Similarly, the REFUND Act, signed in 2021 and in effect since 2023, targets inefficiencies in the healthcare system, specifically focusing on reducing drug waste associated with single-dose vials. By mandating refunds for discarded medication exceeding threshold amounts in outpatient settings, the Act pressures manufacturers to rethink packaging and delivery formats to minimise waste and associated costs.
“These policies incentivise innovations that enhance cost efficiency and demonstrate clear value to payers and providers.”
IMPLICATIONS FOR PHARMACEUTICAL MANUFACTURERS
Both the IRA and the REFUND Act demand a paradigm shift in how pharmaceutical companies approach product development, packaging, pricing and market strategies. These policies incentivise innovations that enhance cost efficiency and demonstrate clear value to payers and providers. To address the REFUND Act, a pharmaceutical manufacturer may consider transitioning to a different vial format, potentially minimising drug waste, or allowing multiple vial configurations to optimise for weight-based dosing.
While these actions may lead to reduced drug waste and accordingly decreased penalties under the REFUND Act, they are generally considered both lengthy and costly endeavours for a pharmaceutical manufacturer to undertake, and accordingly are not initiated easily. Choosing the right primary container and drug delivery device for a drug product and, specifically, considerations for choosing the right infusion pump and digital health platform, can play a critical role in meeting policy requirements and avoiding negative financial implications.
PRICING PRESSURE AND DURABLE INFUSION PUMPS
With the introduction of penalties and price negotiations across a variety of drugs leading to increased pricing pressure and lower margins, drug manufacturers may choose to invest in cost-efficient drug delivery products, with lower costs per dose, shifting away from single-use, fully disposable devices and prioritising reusable systems. Infusion pumps, generally considered durable systems with disposable administration tubing sets, address this pricing challenge, allowing homecare providers and out-patient institutions to use a single robust infusion pump for multiple years, across a variety of therapies.
EITAN MEDICAL: SUPPORTING PHARMA IN A NEW REGULATORY ERA
Eitan Medical’s portfolio of infusion solutions can support pharmaceutical manufacturers as they align with the priorities established by the IRA and REFUND Act.
Sapphire
The Sapphire™ infusion pump platform is the solution of choice for infusion therapy devices across the continuum of care, from pre-acute to hospital and to home. Designed with patient safety in mind, and aiming to improve the daily lives of patients, built-in safety mechanisms are included, along with a simple and intuitive, full-colour touchscreen for fast operation. Smart technology helps infusion providers reduce dosage errors and false alarms. With Sapphire Connect, Sapphire pumps are within reach, transmitting data to the cloud through universal plug-and-play cellular technology. And comprehensive service and support provided by a global network of authorised service centres ensures the infusion providers and patients are at the centre of care.
Avoset
The connected Avoset™ infusion pump transforms specialty pharmacy and home infusions with a compact and simplified technology that enhances the user experience. Remote visibility of infusion data and access to data analytics can help healthcare professionals and infusion providers monitor compliance and identify trends for better care planning. The solution may increase patient access to home infusion and improve quality of life by making home infusion delivery safe, with the potential to allow more patients to receive therapy in the comfort of their own home. The Avoset pump is easy to set up and simple to operate, reducing risk of error with minimal set-up steps, an easily clicked-in administration set and web-based PC programming. Finally, the pump is designed to deliver from a variety of medication containers, including bags, rigid containers and syringes, with further configurations in development.
Eitan Insights
Eitan Insights™, the company’s cloud-based software platform designed to transform infusion management, launched in 2023 and has since won the 2024 National Home Infusion Association innovation award. The platform provides remote visibility to treatment data, along with geolocation of the Sapphire and Avoset pumps, supporting infusion providers in care planning and adherence monitoring. The data presented in the platform eliminate subjectivity, which can help avoid anxiety-provoking troubleshooting, thus helping to improve both patient and caregiver experience. Eitan Insights is built on cutting-edge cloud architecture that uses the latest security features and best practices with state-of-the-art encryption and multi-factor authentication – and it is HIPAA and GDPR compliant (Figure 2).

Figure 2: Eitan Insights, the cloud-based digital health platform.
TAILORED INFUSION SOLUTIONS
Eitan Medical’s software-based pumps are engineered to support accurate infusion therapy, aimed at reducing variability and enhancing outcomes. In parallel, its digital health capabilities provide access to robust evidence of increased operational efficiency, cost effectiveness, proof of treatment and patient adherence. In addition to its commercially available products, Eitan Medical partners directly with pharmaceutical manufacturers, tailoring infusion pumps, sets, accessories and software to meet the needs and challenges of specific drug products, patient populations, geographies and care environments. This can include customised administration sets to meet the specifications of unique primary containers, software and labelling translations to allow introduction into new markets, and complex infusion protocols supported by pump software.
Eitan Medical’s tailored approach also includes aligning pumps and sets to therapy needs, reducing drug transfer steps to minimise wastage and optimising software configurations for specific treatment protocols. The company’s commitment to innovation ensures its solutions not only comply with regulatory requirements but also deliver measurable value to pharmaceutical partners, addressing demands for cost-effective, patient-centric solutions.
INFUSION DATA AND INSIGHTS
Eitan Medical’s connected pumps collect infusion data, not only informing the company as a manufacturer on how its pumps are used – helping to optimise technology, investigate complaints and better support users – but also having the potential to offer pharmaceutical manufacturers real-world evidence on how their drugs are being administered. Access to this data throughout clinical studies can support research efforts and provide aggregated, drug-specific insights. These insights have the potential to serve as never-before-seen evidence for pharmaceutical companies for optimisation of drug product protocols, enhancing patient support programmes and improving adherence strategies.
ENABLING HOME CARE AND DECENTRALISED TRIALS
The shift towards home care and decentralised clinical trials is a significant trend in global healthcare, with pharmaceutical innovators using digital technologies to address the limitations of traditional site-based clinical trials1 and commercialising their drug products in outpatient settings. These models not only reduce costs to the healthcare system as a whole but also improve patient outcomes by providing care in the comfort of a patient’s own home, while significantly reducing the risk of hospital-acquired infections.
With a primary gap of the adoption of both decentralised clinical trials and home-based therapies being the risk of non-adherence to prescribed medications, Eitan Medical’s products are at the forefront of this transformation, with the Avoset and Sapphire pumps designed to support remote monitoring and flexible care delivery, making them ideal to address these challenges. Eitan Insights plays a crucial role in these settings as well, providing remote visibility to treatment data, supporting healthcare providers in the shift towards care at home (Figure 3).

Figure 3: Connected infusion pumps, enabling care at home.
“While the IRA and REFUND Act are US-centric policies, the ripple effects are expected to extend to European pharmaceutical manufacturers and beyond.”
ADDRESSING GLOBAL IMPLICATIONS
While the IRA and REFUND Act are US-centric policies, the ripple effects are expected to extend to European pharmaceutical manufacturers and beyond. Companies operating internationally must adapt to these regulatory shifts, especially as similar trends in drug pricing and waste reduction are expected to emerge in other regions. Eitan Medical’s infusion solutions, with a global distribution presence in over 40 countries, provide a universal framework for compliance and efficiency, ensuring pharmaceutical partners remain competitive across markets.
THE ROAD AHEAD
As the pharmaceutical industry navigates the challenges of drug pricing reforms and regulatory changes, the role of advanced drug delivery technologies and partnerships becomes increasingly critical. Companies must adapt to a regulatory environment that prioritises affordability and efficiency, while continuing to innovate for patient-centric care. Eitan Medical’s proven commercial track record, global presence and comprehensive product offering position it as a trusted partner in this evolving landscape. By using Eitan Medical’s infusion solutions and digital health tools, pharmaceutical manufacturers can strive to not only achieve compliance but also drive innovation, sustainability and efficiency in patient-centred drug delivery.
REFERENCE
- Jean-Louis G, Seixas A, “The value of decentralized clinical trials: Inclusion, accessibility, and innovation”. Science, 2024, Vol 385, Issue 6711.
Previous article
510(k) CLEARED, USER-FILLED MICRODOSING DEVICE FOR OPHTHALMIC AND OTHER APPLICATIONSNext article
DRUG DELIVERY TRENDS FOR 2025
