To Issue 142
Citation: Gallina S, “Engineering Advances in Needle Geometry to Accommodate Viscous Biologics”. ONdrugDelivery, Issue 142 (Feb 2023), pp 80–82.
Silvia Gallina discusses the challenges faced in administering highly viscous biologics to patients via subcutaneous injection, and how Stevanato Group’s new special thin-wall needles are able to tackle them.
In many ways, drug delivery can be regarded as a science of managing variables. The variables in question might be influenced by, for example, evolving market requirements, advances in technological capabilities or the emergence of breakthrough treatments. Alternatively, the status quo might be challenged by the continual push to innovate around patient needs, whether in terms of maximising therapeutic benefit, reducing exposure to risk or providing a better drug delivery experience overall.
“Due to the complex structure of biologics, their physical and chemical stability is a constant concern due to the sensitivity of their formulations, with stimuli such as temperature posing a risk to their structural integrity.”
One area where such challenges have given rise to new and evolving variables is in the parenteral delivery of biologics and the associated requirement to handle high-viscosity formulations. Another example can be seen in the healthcare industry’s transition towards increased levels of drug self-administration in the home rather than in traditional clinical settings. At the intersection of these two examples, further challenges have arisen in the effort to deliver viscous biologics via patient-controlled injection devices.
This article reflects on these trends, exploring the questions they pose in relation to drug delivery and the associated issues that can arise for patients. It then goes on to explain how Stevanato Group is supporting pharma companies in responding to these challenges, specifically focusing on the development of a special thin-wall needle that fulfils the growing need for managing the delivery of viscous injectable formulations within autoinjector devices.
RESPONDING TO THE GROWTH OF THE BIOLOGICS MARKET
The biologics market is expected to continue to grow in the coming years. Differing market projections vary in the extent of this growth, but there is general acceptance that sales of biologic compounds will outstrip those of small molecules in the near future, possibly by as much as US$120 billion (£101 billion) by 2027.1
Digging deeper, monoclonal antibodies are expected to make up the most significant share of biologic sales, while high-growth areas include gene therapies and gene-modified cell therapies. Biosimilars are also forecast to register sustained double-digit growth as exclusivity arrangements end, triggering a rise in competition, demand and uptake over time.
Looking at the properties of these drugs, there continue to be limitations when it comes to administration. Due to the complex structure of biologics, their physical and chemical stability is a constant concern due to the sensitivity of their formulations, with stimuli such as temperature posing a risk to their structural integrity. They are also susceptible to degradation through processes such as aggregation and denaturation.
Taken together, these characteristics have historically made parenteral administration via injection or intravenous (IV) infusion the preferred delivery methods to maximise the bioavailability of biologics. Parenteral delivery does not come without compromises, however. For the patient, there is the need to rely on a healthcare professional (HCP) to administer IV infusions, which can necessitate visiting a clinic, with the associated personal burden for patients and caregivers, particularly where frequent dosing is required.
“With a thinner wall, the internal diameter and volume of the needle chamber are increased to allow the formulation to flow more easily without increasing the needle’s overall diameter.”
Self-administration via subcutaneous (SC) injection, including the use of autoinjectors, provides an alternative solution here. However, injection opportunities are limited by the volume of drug acceptable to the patient, which is generally acknowledged to be between 1 and 2 mL, although more recently this ceiling has been increased to around 3 mL.
At a fundamental level, maintaining SC injection volumes within these thresholds may require reformulation, resulting in the creation of high-concentration formulations. However, with increasing concentrations come increasing levels of viscosity, which can have implications for injection force – and therefore injection time – unless the gauge of the needle is increased. As such, a proper evaluation of the container must be performed from the earliest stages of drug development in order to meet injection time requirements, while also keeping focus on the overall patient experience.

Figure 1: The enlarged internal channel diameter of sTW needles enables a smooth delivery of highly viscous biologic solutions without the need for higher gauge needles.
LIMITING INJECTION FORCE AND TIME OPTIMISING THE PATIENT EXPERIENCE
Many factors contribute to the overall sensation of pain associated with an injection, from the location on the body to the injecting action and the nature of the formulation, including make-up and volume. While it is not possible to manipulate and resolve all these variables, certain key elements can be addressed to help limit pain, including the thickness of the needle wall.
With a thinner wall, the internal diameter and volume of the needle chamber are increased to allow the formulation to flow more easily without increasing the needle’s overall diameter. This means that the required glide force and injection time can be reduced for viscous formulations without necessitating the selection of a higher gauge needle, and thereby reducing the pain experienced by the patient.
This has clear benefits for the patient experience, which can encourage improved adherence. It also helps address potential mechanical challenges associated with autoinjector devices, where higher break-loose and glide forces can lead to an increased risk of breakage and, therefore, drug wastage.
Stevanato Group, due to the intimacy of its pharma relationships and its deep understanding of patient needs, is always evaluating how progress can be realised in relation to technical challenges such as this through advances in design, engineering and chemical science. As a result, Stevanato Group has developed a special thin-wall (sTW) needle that optimises the delivery of viscous biologics without requiring the use of higher gauge needles (Figure 1), thereby satisfying autoinjector device requirements and enhancing the patient experience.
Available in 27G and 29G, the 0.5 inch needles with five-bevel tips are manufactured from AISI 304 stainless steel and have been designed to meet the requirements of Stevanato Group’s high-performance Nexa® and Alba® container platforms in terms of dimensional performance, cosmetics, penetration and breakage. sTW needles are currently applicable to 1 mL long and 2.25 mL syringe formats, with the main target being autoinjector-based biologics.
THE CORRELATION BETWEEN NEEDLE DIAMETER AND GLIDE FORCE
To analyse the comparative performance of the sTW needles against standard thin-wall (TW) needles, a series of in-house tests was undertaken by Stevanato Group. These studies were designed to gather data on the quality attributes of sTW needles and the characteristics they display in combination with rigid needle shields (RNSs) and when deployed within an EZ-fill® configuration.
The tests were carried out by trained personnel according to approved methodologies and with a statistically valid number of randomised samples. Performance was verified after three and six months based on accelerated and real-time conditions according to ASTM F-1980 and ICH Q1A guidelines. Tests on breakage, elasticity, flexural strength and glycerine colouring were performed in accordance with ISO 9626 and Korean Pharmacopeia standards.
For the EZ-fill® syringes incorporating the sTW needles, this included testing for needle axiality, hooks needle and cosmetics. Furthermore, performance remained within Nexa® specifications for the sTW after the three- and six-month storage periods. It can therefore be concluded that an enlarged channel diameter, as provided by sTW needles, does not impact performance in terms of cosmetics or mechanical resistance.
Subsequent testing was carried out to analyse sTW syringe glide force reduction when a highly viscous solution is present. This was achieved by filling 1 mL long syringes 27G TW and 27G sTW with different solutions: 1 cP and 15 cP glycerol solutions. Syringes were then tested for glide force at a consistent rate of 100 mm per minute and 250 mm per minute to simulate the speed observed during manual and autoinjector injections respectively.
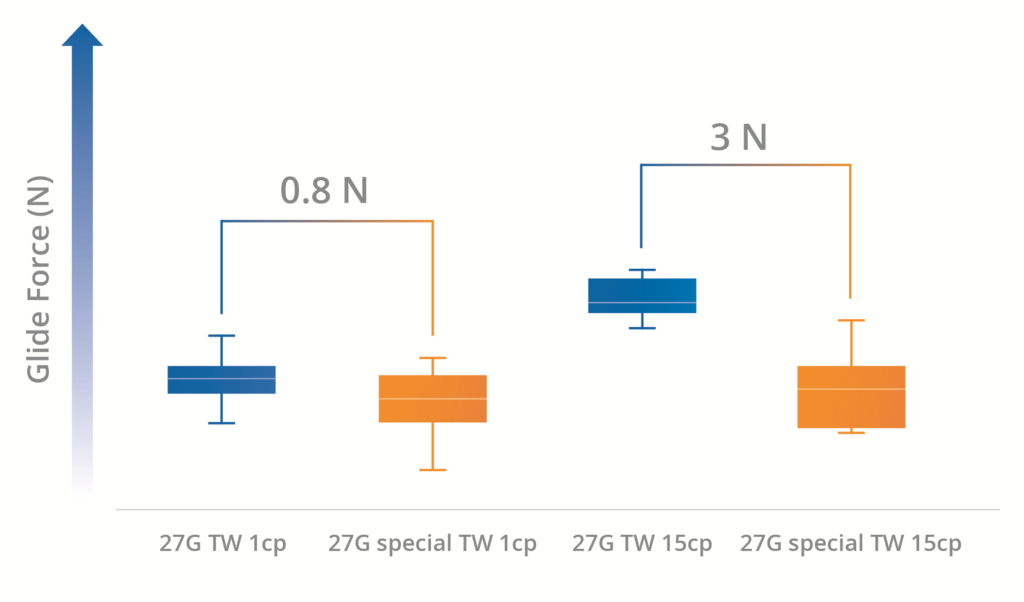
Figure 2: Glide force of 1 mL long Nexa® syringe with 27G TW and 27G sTW ½” needle filled with 1 cP and 15 cP solutions at 100 mm/min.
As shown in Figures 2 and 3, in every case, the sTW needle registered a lower glide force than the counterpart standard TW needle. Furthermore, it could be seen that the reduction in glide force became more significant as the viscosity of the solution increased – a 3 N glide force reduction with the 15 cP solution through a sTW needle (Figure 2). Additionally, the effect is also more significant at higher plunger stroke speeds – a 6 N glide force reduction with the 15 cP solution through a sTW needle at 250 mm/min (Figure 3).
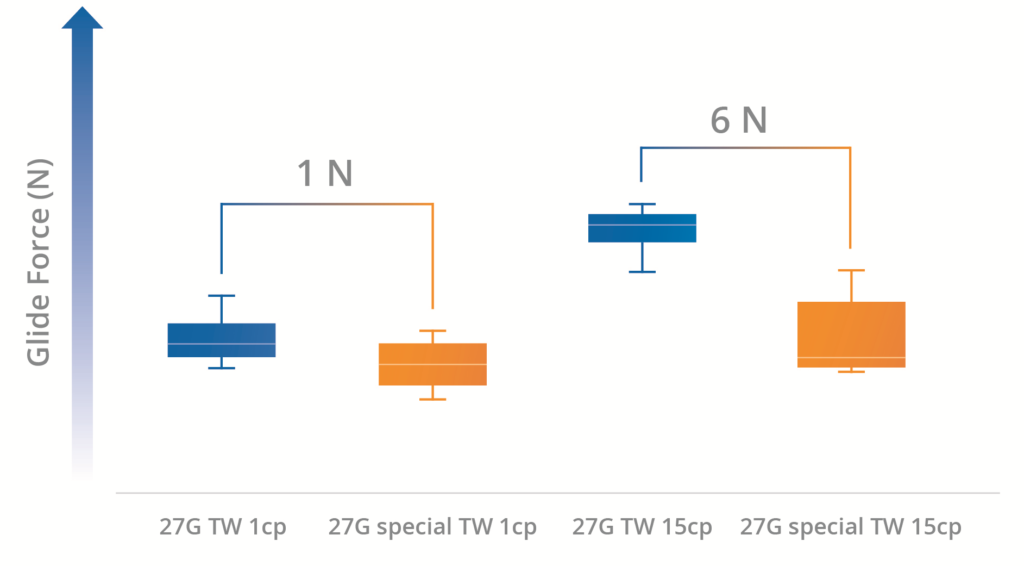
Figure 3: Glide force of 1 mL long Nexa® syringe with 27G TW and 27G sTW ½” needle filled with 1 cP and 15 cP solutions at 250 mm/min.
AN UNCOMPROMISING APPROACH TO THE MANAGEMENT OF VARIABLES IN DRUG DELIVERY
As these tests show, the sTW needle for the Nexa® and Alba® container platforms, using both 1 mL long and 2.25 mL syringe formats, can reduce the glide force for highly viscous solutions. As such, sTW needles avoid the need to move to a higher needle gauge to accommodate biologic formulations, which would have a detrimental effect on the patient experience.
As the biologics segment continues to grow in size and influence, and pharma companies look to deliver these life-changing drugs via patient-friendly devices and in patient-centric settings, new challenges will continue to arise. Managing all the variables at play can sometimes be a case of balance and compromise, but, as the development of the sTW needle shows, sometimes it is a challenge that can be answered directly through engineering expertise and innovative thinking.
REFERENCE
- “Future of Pharma – Looking Ahead to 2022”. GlobalData, Mar 2022.
Previous article
BEING PREPARED FOR PHARMA INNOVATION IS KEY IN 2023Next article
A TALE OF TWO SPRINGS
