To Issue 138
Citation: Calderwood G, Ganea R, Fuensalida Pantig G R, “Enlarging the Volume of Autoinjectors: Traversing Injection Boundaries”. ONdrugDelivery, Issue 138 (Oct 2022), pp 26–32.
Gary Calderwood, Raluca Ganea and Gene Rhode Fuensalida Pantig review how the parenteral space has transformed over the past decade and reports how the company’s portfolio of autoinjector technologies has evolved to deliver subcutaneous parenteral drugs of varying formulations, including higher administration volumes.
The medtech industry is at a crossroads, presented with the opportunity to actively participate in and redefine what kind of combination products will emerge and reach the hands of patients in the near future. Prefilled syringe (PFS)-based mechanical autoinjectors have been smoothly integrated in the co-development process between pharma and medtech, enabling patients to self-administer conventional biologics and biosimilars subcutaneously (SC). However, as the R&D formulation pipeline sees volumes and viscosities beyond what it has previously had to deal with, what device technologies does the medtech sector have to offer?
“While considerable progress has been made in formulation technology development, it is of equal importance to develop solutions that put the patient experience at the centre of any new delivery system in order to advance parenteral drug delivery.”
A REVIEW OF THE RECENT DEFINING MOMENTS IN PARENTERAL DRUG DELIVERY
The current parenteral drug landscape contains 112 autoinjector products (single use, including emergency devices) addressing various chronic diseases. These areas include metabolic, autoimmune, inflammatory and systemic conditions, to name but a few. The latest five-year average for the US FDA’s new molecular entities and biologics approvals currently stands at 51 per year. Ten years ago, it was 24 molecules per year. During this time period, notable advancements have been made in formulation science that have facilitated the SC delivery and absorption of biological products, the list of which now includes chemotherapeutic agents – something that has never been seen before.
At this rate of development, there is a possibility to expect more biopharmaceutical developments to shift in the direction of SC administration. Of the many approvals across the decade, there are a few notable ones. For example, a breakthrough therapy for atopic disorders was first approved in the US and EU in 2017 and, within the space of just a few years, this same biologic entered the self-injection space.
This is how, in 2020, SHL Medical found itself as the device developer of one of the world’s first PFS-based mechanical autoinjectors in the larger volume range (≥2 mL). At that time, it was not common for a true 2 mL fill autoinjector combination product to be launched on the market – even more so for systemic therapy. This suggests that there is potential to further expand the use of autoinjectors in SC therapy.1–5
EXPLORING AUTOINJECTORS BEYOND 2 ML
In 2018, the SC Drug Delivery and Development Consortium was convened with the goal to identify solutions and raise awareness of the issues and gaps in the high-dose/large-volume drug formulations market. The Consortium aims to fully explore the potential of the SC route of administration. It is notable that, since its inception, the Consortium has consisted of leading members of the biopharma and R&D communities. It is therefore recommended that representatives of the medtech industry take part in these discussions and share their thought leadership to further advance the technology within the drug delivery space.
The consortium has developed and reported on eight problem statements that highlight a composite of actionable challenges in SC drug delivery. Ranked in the order of priority, high-dose/volume SC technology development is reported to be the first, followed by bioavailability, immunogenicity and then patient preference (Table 1). The focus of this article is on problem statements 1 and 4.
| Order | Subject | Condensed Problem Statement |
| 1 | High-Dose/Volume SC Technology Development |
Misguided industry perception of what is possible in the development of large-volume (>2 mL) and high-dose SC device technologies |
| 2 | Bioavailability | Unpredictable and variable bioavailability of biologics |
| 3 | Immunogenicity | Lack of consistency across industry and understanding of untoward SC immunogenicity and the relevant test method to evaluate such a phenomenon |
| 4 | Patient Preference: IV vs SC | Patient preferences are unprioritised and not clearly understood in order to identify optimal trade-offs between IV and SC product design attributes |
| 5 | Clinical Trial Strategy | Unclear understanding of when to initiate SC clinical trials |
| 6 | Payer Preference | Unclear understanding of the value of SC injections beyond the price point |
| 7 | Patient Experience & Discomfort | Need to understand further how the patient experience impacts patient preferences and define quantifiable metrics to assess injection |
| 8 | Patient-Physician Interactions | Unclear understanding of the role of physicians in educating patients about the benefits of SC injections and the impact on overall patient preferences for IV or SC |
Table 1: Problem statements – condensed for this article – in SC drug delivery developed by the Consortium. First reported in the review article titled “Accelerating the development of novel technologies and tools for the subcutaneous delivery of biotherapeutics” published in 2020.6
Considering the steady development of complex biologics, formulation technologies and the identified advantages of treatment self-administration, now is the time for pharma and medtech to explore autoinjectors capable of delivering doses beyond 2 mL. For SHL, it is important to provide a better understanding of current advances in autoinjector technologies in order to shift perceptions of their key role in next-generation SC drug development and delivery.6
A CARTRIDGE-BASED SOLUTION TO ENABLE AUTOINJECTORS BEYOND 2 ML
The overwhelming majority (>98%) of combination products in PFS-based, single-use autoinjectors have an injection volume of 1 mL or less. However, it would be negligent not to consider that current biopharmaceutical trends and pharma pipeline molecules suggest a growing need for higher-capacity drug delivery technologies. This is essential for enabling a better and more comprehensive health and medical landscape. As the healthcare market evolves, device technology development remains central to delivering SC parenteral drugs that require higher administration volumes.7
While considerable progress has been made in formulation technology development (such as particle engineering, transiently altering the SC space, etc), it is of equal importance to develop solutions that put the patient experience at the centre of any new delivery system in order to advance parenteral drug delivery. Patient preference is one of the consortium’s top four challenges, which reinforces the value of therapeutic self-administration and the advantages of enabling drug delivery devices, like autoinjectors, particularly those that emphasise ease of use.
Conventionally, autoinjectors are developed around a PFS, which, with its staked needle, can be designed into two- or three-step self-injection devices that do not expose the user to the needle. In comparison, cartridge-based pen injectors traditionally require the user to manually attach the needle to the device before injection. This additional user step may pose a risk of foreign particle contamination and needle-stick injury. In the context of therapeutic self-injections, operational safety is highly important to facilitate medication adherence and improve the patient experience. Considering this, SHL has developed an innovative mechanism called “Needle Isolation Technology” (NIT®) that addresses the challenges associated with cartridge-based injection systems.
Based on a pre-installed needle housed within the cap of the device, NIT technology eliminates the need for users to manually attach the needle. With NIT, users simply unscrew the cap to engage the needle with the cartridge prior to injection, thereby opening the fluid path and allowing the injector to prime automatically. NIT makes it possible for the cartridge to behave like a traditional PFS with a staked needle; the device includes complete needle covering and shielding before and after injection. Additionally, the technology supports complete control of cannula gauge and length to enable target injection time and depth. The removal of an apparent design attribute trade-off between PFS- and cartridge-based autoinjectors has allowed NIT to pave the way for the possibility of safe and intuitive large-volume/high-dose autoinjectors.
Prior to the establishment of the consortium, the first cartridge-based autoinjector built with NIT was approved by the FDA in 2017. The autoinjector, which is the second-generation device of SHL’s pharmaceutical partner’s GLP-1 receptor agonist, offers its patients a more convenient injection experience. Since its commercialisation in 2018, the combination product has delivered millions of doses to patients worldwide. Regarding patient experience and safety, the data currently available to SHL demonstrate how the market-proven NIT subassembly has successfully facilitated the safe and effective self-injection experience of patients.8,9
GOING BIG: A TECHNOLOGY TO EXPLORE LARGE-VOLUME AUTOINJECTORS
The inclusion of NIT in an autoinjector opens pathways for increased dose volumes and viscosities, as well as the accommodation of lyophilised drugs, suspensions and other specialty formulations. If limited to PFSs as the primary container, the delivery of high doses will require reformulating the product, in turn raising concerns about stability, particle aggregation and the resultant viscosity.
Recently, treatments in the oncology area have been exploring the transition from the intravenous (IV) to the SC route of administration through enzyme-assisted drug formulations, further opening the possibility of cartridge-based autoinjector combination product development. When considering the treatment burden on the patient, data available to SHL suggest that there is a preference towards delivering a drug product using a single device, rather than multiple. SHL performed comparative literature studies to produce this reasoning.
Additionally, the development of primary containers with volumes greater than 2 mL and the infrastructural maturity of the associated fill/finish technology opens pathways for the appropriate large-volume device technology. To enable patient self-administration in circumstances where a PFS-based autoinjector seems infeasible for single-device injection, SHL has developed the Maggie® device technology.10–12
The Maggie device, built with NIT, was initially developed with a standard 3 mL cartridge. It was designed to address the technology gap between PFS-based autoinjectors and on-body patch pumps, effectively expanding the potential delivered dose volumes of autoinjectors to 3 mL. The architectural design of the device and its NIT subassembly allows the needle to be permanently hidden, from the moment when the patient unscrews the device cap up until the device is disposed of. This is intended to alleviate preconceived patient concerns associated with needle-based injections.
In 2019, a human factors study was performed by SHL and its research partner to understand the operational usability of the Maggie device. Using a standard protocol, device-experienced and device-naïve participants were given a Maggie autoinjector to use and were then asked to rate the ease or difficulty of operation (where 7 is highest and 1 is lowest). Nearly all participants reported a high regard for Maggie when it came to its ease of operation, indicating a very user-friendly device (Figure 1). Furthermore, this study has enabled SHL to collect user feedback regarding the device’s strengths and opportunities for improvement, and has found that no significant design improvement is needed for the device to successfully function. The study highlighted the intricate design engineering work that SHL has invested in to address the technological gaps in the drug delivery sector.13
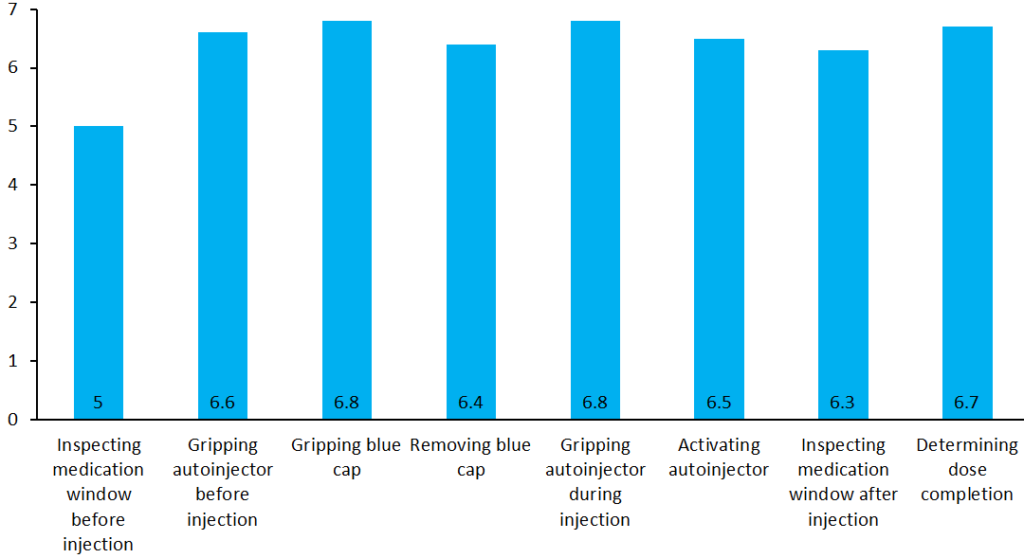
Figure 1: Statistical summary of a Maggie human factors study conducted in 2019. After using the autoinjector, nearly all participants highly regarded Maggie for its ease of operation, indicating a very user-friendly device. Evaluations were made on a scale where 7 is highest and 1 is lowest.14
With a strong future outlook, SHL further challenged the notion behind the limits of SC autoinjectors. SHL notes that explorations between medtech and pharma are currently lacking due to existing perceptions of what is possible in the development of large-volume SC devices. With the right technical research and collaboration in the combination product development process, autoinjectors should be seen for their expanded large-volume/high-dosing utility – formally closing the technology gap between PFS-based autoinjectors and on-body patch pumps. In response to this need, SHL recently launched Maggie 5 mL – the larger-volume variety within the Maggie family of devices. This larger-volume version leverages the lessons gathered from its predecessor and retains the two-step injection handling sequence (Figure 2).
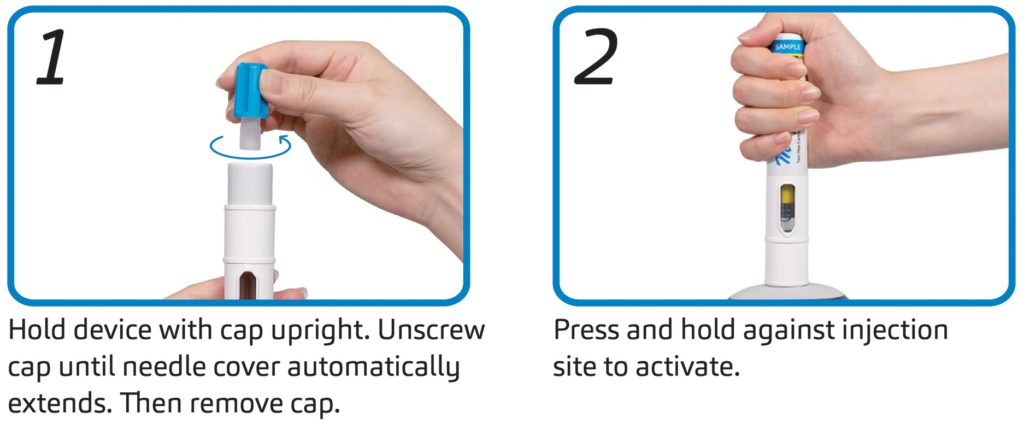
Figure 2: Basic handling instructions for Maggie 5.0 – a two-step autoinjector and the newest addition to the Maggie family of devices.
Currently available scientific research indicates that biological therapeutics are shifting from traditional IV administration towards SC administration to reduce costs, time of delivery and adverse systemic effects. The ultimate goal is to increase patient quality of life and treatment adherence. Preliminary available information indicates that the SC tissue can be limited in its ability to handle fast injections of large volumes, resulting in varying degrees of leakage (sometimes called “wet injections”), site reaction and patient discomfort. Although there might be some limitations, SC delivery of large volumes seems to be feasible and tolerable based on currently available research.15–17
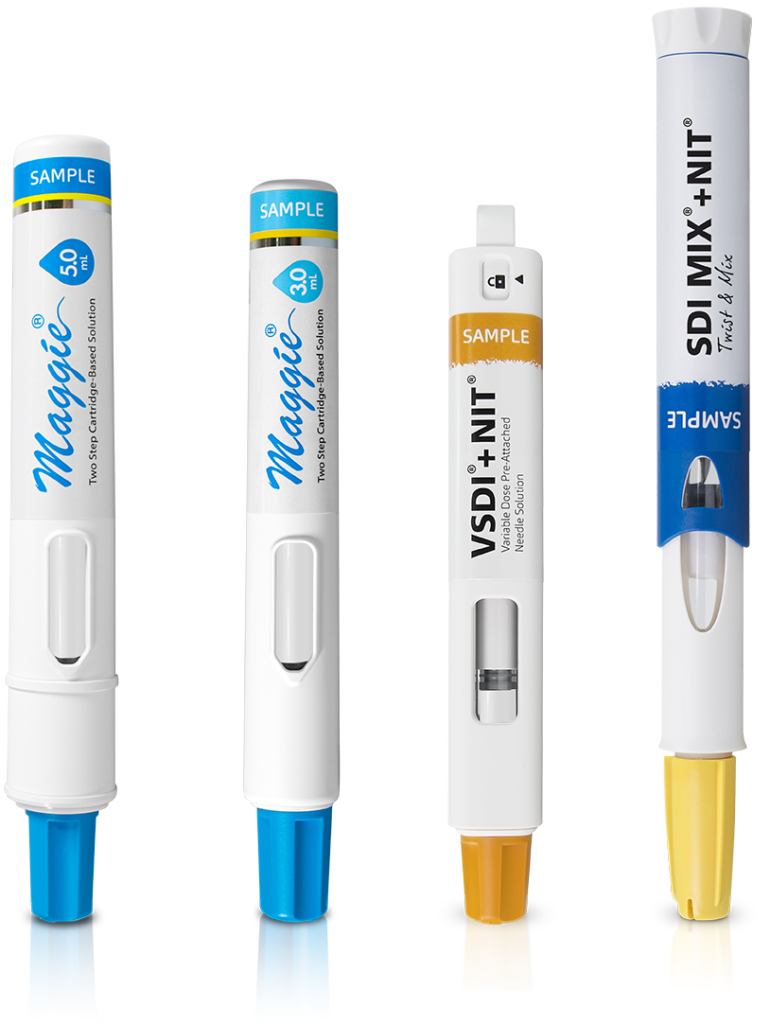
Figure 3: Designed to address the needs of varying molecules, SHL’s NIT® family of devices includes autoinjectors for larger-volume, lyophilised and other specialty formulations.
At present, several technologies have been proposed to overcome some of the mentioned limitations and these are able to minimise intermolecular interactions to reduce the viscosity of high-concentration formulations, to form fluid suspensions or to modify the SC space to facilitate the delivery of a larger volume of fluid, such as transient permeation.12
SHL has also undertaken preliminary usability work on the feasibility of longer holding times for autoinjectors. In terms of holding stability over simulated injection times of up to 60 seconds, the study showed no statistically significant change in relative positioning, orientation or needle cover engagement of the device with the injection site. The composite of these elements serves as SHL’s guiding principle to explore cartridge-based autoinjectors – through SHL product offerings that are in various stages of development – with its pharma partners even further (Figure 3).
“True to its modular product design, expanding the Molly’s design attributes to improve device performance is an active pursuit – SHL has seen technical progress in the delivery of higher-viscosity drugs, while also maintaining the same device geometry.”
PARALLEL DEVELOPMENTS AND FUTURE OUTLOOK
As SHL advances the technological feasibility of large-volume drug delivery, there remains a focus on continuing to enable conventional device needs across various disease areas. The Molly® autoinjector device technology continues to meet with regulatory approval and commercial success, made possible by a modular manufacturing set-up that allows SHL to manage multiple simultaneous device projects, streamline processes and enable faster development timelines. Recently, the modular Molly platform has been supporting the high production forecast demand for a combination product approved in 2021, which has far exceeded SHL’s previous levels of device delivery to the market. This is a true testament to Molly’s scalability and SHL’s responsiveness to the ever-changing annual production needs of a combination product.
True to its modular product design, expanding the Molly’s design attributes to improve device performance is an active pursuit – SHL has seen technical progress in the delivery of higher-viscosity drugs (≥ 25 cP), while also maintaining the same device geometry. The Molly autoinjector is now compatible with 8 mm needle PFSs, offsetting injection constraints in PFS-based mechanical autoinjectors. This increased freedom for injectable drug delivery is also supported by wider pre-set viewing window options that are optimised for different injection volumes (Figure 4). SHL has redefined the idea behind conventional autoinjectors through platform modularity, and the Molly autoinjector technology continues to evolve to address the ever-changing market needs.

Figure 4: Enabled by platform modularity, the Molly autoinjector technology is always evolving and allows for various customisations according to industrial design, branding and commercialisation strategy, formulation and patient requirements.
For SHL, the key advantage to having a design and manufacturing infrastructure built upon platform concepts is that such an asset investment philosophy can be leveraged for its Maggie technology offering. The Maggie device family is intended to be a large-volume autoinjector platform that addresses customer requirements on priming and drug delivery volumes, as well as injection time, cannula size and injection depth. Just as the modular Molly is the platform of choice for more than a dozen global pharma companies, the goal for the Maggie family of devices is to become the industry platform of choice to enable patients’ independence in disease areas in large-volume/high-dosing therapy as yet unseen in the self-injection space.
REFERENCES
- IQVIA data, 2022.
- Mullard A, “2021 FDA Approvals”. Nat Rev Drug Discov, 2022, Vol 21(2), pp 83–88.
- “Enhanced Hyaluronidase-based Drug Delivery”. Biopharm Deal, 2018, Vol 12(3), p B12.
- Hodgson J, “Refreshing the Biologic Pipeline 2020”. Nat Biotechnol, 2021, Vol 39, pp 135–143.
- Stewart J, “Dupixent FDA Approval History”. Drugs.com, Jun 2022.
- Collins DS et al, “Accelerating the Development of Novel Technologies and Tools for the Subcutaneous Delivery of Biotherapeutics”. J Control Release, 2020, Vol 321, pp 475–482.
- IQVIA data, 2022.
- “Autoinjector Designed by SHL Receives 2019 Medical Design Excellence Awards”. SHL Press Release, Jun 2019.
- SHL Medical internal data, 2022.
- Pivot X et al, “Preference for Subcutaneous or Intravenous Administration of Trastuzumab in Patients with HER2-positive Early Breast Cancer (PrefHer): An Open-Label Randomised Study”. Lancet Oncol, 2013, Vol 14(10), pp 962–970.
- SHL Medical literature review, unpublished, 2022.
- Badkar AV et al, “Subcutaneous Delivery of High-Dose/Volume Biologics: Current Status and Prospect for Future Advancements”. Drug Dec Devel Ther, 2021, Vol 15, pp 159–170.
- SHL Medical human factors study, unpublished, 2019.
- SHL Medical human factors study supplementary materials, unpublished, 2019.
- Doughty DV et al, “Understanding Subcutaneous Tissue Pressure for Engineering Injection Devices for Large-Volume Protein Delivery”. J Pharm Sci, 2016, Vol 105(7), pp 2105–2113.
- Woodley WD et al, “Clinical Evaluation of an Investigational 5 mL Wearable Injector in Healthy Human Subjects”. Clin Transl Sci, 2021, Vol 14(3), pp 853–869.
- Woodley WD et al, “Clinical Evaluation of Large Volume Subcutaneous Injection Tissue Effects, Pain, and Acceptability in Healthy Adults”. Clin Transl Sci, 2022, Vol 15(1), pp 92–104.

