To Issue 153
Citation: Morris W, “Environmental Drivers for Change in Drug Delivery Devices”. ONdrugDelivery, Issue 153 (Oct/Nov 2023), pp 56–59.
Will Morris discusses the positive changes that have occurred when it comes to environmental sustainability in the medical industry, in the context of examining what more can be done.
When designing medical devices, it is important to focus on the positive impact that they have on people’s health, but not to ignore the negative impact that they can have on the environment. It is a sobering experience to visualise the quantity of devices that are being designed, the scale at which they will be manufactured, and the resultant magnitude of carbon dioxide equivalent (CO2e) emissions and non-recyclable plastic produced.
“With nearly half a tonne of municipal waste being produced per person, the EU is aiming to introduce new waste output measures for both prevention and reduction.”
Globally, healthcare is responsible for two gigatonnes of CO2e annually – 4.4% of global emissions. This is not an insignificant amount; it is equivalent to the CO2e emissions produced in powering every single home in the US with electricity for one year. So, it is important not to “greenwash” the industry. However, it is worthwhile reflecting on the positive changes that have occurred when it comes to environmental sustainability in the medical industry, in the context of examining what more can be done.
SYSTEM CHANGE
UN Sustainability Goals
In 2015, all United Nations (UN) member states adopted the 2030 Agenda for Sustainable Development. This ambitious programme consists of 17 Sustainable Development Goals (SDGs), which are an urgent call for action by all countries and organisations. There are a number of goals directly relating to environmental impact. The SDG 2030 agenda encourages all countries to take concrete actions and adopt policies that align with the SDGs to promote a more sustainable and prosperous world for all.
EU Circular Economy
Europe is committed to its vision for a circular economy and its plans to achieve climate neutrality by 2050. The European Union’s (EU’s) Circular Economy Action Plan details initiatives to make sustainable products the norm in the EU, empower consumers, ensure less waste and make circularity work for people, regions and cities. This is arguably one of the biggest experiments towards achieving a sustainable and prosperous economy.
The circular economy will have an impact on a variety of medical devices, either directly or indirectly. Electrical and electronic equipment is one of the fastest growing waste streams in the EU. Electronic products that are to be placed on the EU market will be designed for longevity, reuse, repair, update and, ultimately, recycle. This will have an impact on connected healthcare products, wearables, diagnostic devices and electronic drug delivery products.
“Some organisations have released new devices and schemes to combat factors such as polluting propellants, excessive material use, modular electronic add-ons and end-of-life device management.”
Single-use non-recyclable plastic products will be phased out wherever possible. By 2050, plastics could account for 20% of oil consumption and 15% of greenhouse gas emissions – and there could be more plastic in our oceans than fish! That is why eco-design will be prioritised, striving for devices to last longer and/or be easily recyclable.
With nearly half a tonne of municipal waste being produced per person, the EU is aiming to introduce new waste output measures for both prevention and reduction. Appropriate medical waste disposal is complex, and it will be interesting to see what future legislation various markets will adopt to cater for potentially hazardous contaminated medical waste.
The NHS Impact
The UK NHS has committed to reaching net zero by 2040 for the emissions it directly controls – and by 2045 for the emissions it influences. Since the publication of its “Delivering a Net Zero NHS” report, the NHS England board has approved a road map to help suppliers align with its net zero ambitions between now and 2030:
- April 2022: All NHS procurements will include a minimum 10% net zero and social value weighting.
- April 2023: For all contracts above £5 million per annum, the NHS will require suppliers to publish a carbon reduction plan (CRP) for their UK Scope 1 and 2 emissions and a subset of their Scope 3 emissions as a minimum.
- April 2024: The NHS will extend the requirement for a CRP to cover all procurements.
- April 2027: All suppliers will be required to publicly report targets and emissions, and publish a CRP for global emissions aligned to the NHS net zero target, for all their Scope 1, 2 and 3 emissions.
- April 2028: Carbon foot printing will be required for individual products supplied to the NHS. Exact scope and methodology to be decided.
- ~2030: Access to NHS contracts will only be available to those who demonstrate their progress through published progress reports and continued carbon emissions reporting through the Evergreen sustainable supplier assessment.
Figure 1: The ISO 14040:2006 lifecycle assessment.
Legislation
Restriction of Hazardous Substances in Electrical and Electronic Equipment (RoHS); Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH); and the Waste Electrical and Electronic Equipment (WEEE) Directive are significant EU regulations that have been implemented to address environmental and health concerns related to hazardous substances in products and chemicals, and to set rules for the proper collection, recycling and treatment of WEEE. Additionally, the introduction of ISO 14040:2006 enables the industry to better understand and address the environmental impacts of products (Figure 1).
One of the most notable environmental actions regarding hazardous substances was taken in the late 1980s with the Montreal Protocol. At an international level, it was recognised that chlorofluorocarbons (CFCs), a propellant commonly used in pressurised metered dose inhalers (pMDIs), contained ozone-depleting substances. As such, timelines were set to phase out CFC-based inhalers, which are now being switched to hydrofluoroalkane (HFA)-based inhalers.
Even more positive change is beginning within the inhalation sector, where MDI propellants are again changing to HFC 152a or HFO 1234ze(E), propellants that have lower global warming potential (GWP) than their currently used counterparts. In the UK, there is also a stronger push towards dry powder inhalers (DPIs), a format of drug delivery that has a GWP an order of magnitude lower than pMDIs. However, pMDIs are still required as an option, as DPIs cannot typically be used by all patients. It is clear, however, that there is accelerating positive change in this sector, which is likely to affect the trajectory of other types of drug delivery devices as well.
MANUFACTURER CHANGE
Many organisations are working hard to reduce their environmental impact. Some companies have publicised their alignment with their priority SDGs, while others have published their own sustainability initiatives.
In the inhalation sector, many organisations are working hard to achieve not only the SDGs but also their own sustainability initiatives (some actually coming online before the 2015 SDG programme). Some organisations have released new devices and schemes to combat factors such as polluting propellants, excessive material use, modular electronic add-ons and end-of-life device management.
The industry is also seeing more modular and reusable electronic devices, such as Nemera’s Symbioze on-body injector and Owen Mumford’s UniSafe reusable autoinjector. The ability to segregate a device’s mechanical elements from the drug pathway and/or bodily fluid contact has enormous potential for a lot of existing combination products. The key aspect is ensuring that additional complex user steps are not required for preparation of the device during and after use.
The way in which devices are designed has a significant impact on their carbon footprint. YpsoMed has developed the YpsoMate Zero, the world’s first zero-carbon-emission autoinjector. Many other organisations are looking to take this further and are developing drug delivery devices that eliminate polluting plastics, reduce their plastic use or are made from a mono-material solution.
There are also device platforms for pharmaceutical companies to select from, rather than designing their own bespoke device. Furthermore, there’s even collaboration between multiple contract development and manufacturing organisations to produce the same components and devices to further improve economies of scale, and potentially lower environmental impact.
There are several end-of-life device management programmes. GSK’s Complete the Cycle programme (2012) resulted in the recovery and recycling of two million inhalers, saving an estimated equivalent of 8,665 cars’ emissions in one year. Chiesi’s Take AIR recycling programme (2021) enabled any inhaler brand and type to be recycled by post. And Novo Nordisk’s PenCycle programme allowed users to recycle their empty drug delivery pens via selected stores, community pharmacies and at-home collection services.
The success of these initiatives has been mixed, as it depends on a variety of complex factors. The environmental efficacy of a scheme needs to be regularly evaluated to ensure that it has a net positive impact regarding the act of recycling and the infrastructure/logistics required versus disposal and incineration. As seen by some of the early pilot studies, these initiatives require thorough planning, complex logistics and – the most difficult challenge – a change in human behaviour.
“Medical device users play a crucial role in driving positive change for medical devices to be more environmentally friendly.”
PEOPLE CHANGE
Medical device users play a crucial role in driving positive change for medical devices to be more environmentally friendly. In the consumer sector, almost every product has promotional marketing regarding the product’s “green” credentials – whether a shampoo bottle is made with post-consumer recycled plastic or shoes are delivered with plastic-free packaging. This marketing is there to drive eco-conscious people to make informed purchasing decisions. In the healthcare sector, the hope is that consumers will opt for medical devices that are designed with environmental considerations in mind, providing it doesn’t make their personal health management more painful, costly or complex.
Healthcare professionals (HCPs) are now being asked by their patients, “Which of these devices is most sustainable?” This question would have been impossible to answer years ago, but now decision aids are being produced that enable GPs to discuss complex matters with their patients, such as which inhaler has the lowest carbon footprint, often with comparable equivalents, such as a journey in a petrol car or the CO2e of baking a loaf of bread.
Increased at-home care will play a significant role in driving more sustainable drug delivery solutions. Users will naturally try to maximise the use of their devices to save on the cost of buying a new device or the effort of travelling to their local pharmacy. Patients will start to realise and voice their concerns regarding the wastefulness of their drug delivery devices. There will be recognition that the syringe is the only important element to dispose of responsibly – the complex mechanism to deliver the drug could be reloaded and used again if allowed by the device’s design, or at least could be separated into a recyclable waste stream.
All these factors will also be driven from the complexity of appropriate disposal. In the inhalation sector, many people who use pMDIs are shocked to hear that they are not allowed to separate the plastic body from the canister and place them into recycling. Disposal of medical waste can be confusing, therefore, expect to see developments in clarity of appropriate waste disposal instructions, techniques to limit wasteful disposal and strategies to eliminate disposal.
CONCLUSION
In the 30-plus years since the adoption of the Montreal Protocol, there has been significant positive change regarding making drug delivery devices more environmentally friendly (Figure 2). However, there is still so much more opportunity for improvement. It is clear that it’s not just a single-sided argument; legislators telling manufacturers what to do, and then forcing people to only use certain types of drug delivery devices. Rather, it is a complex three-way discussion that is now being informed by real-world data and both bottom-up and top-down information exchange.
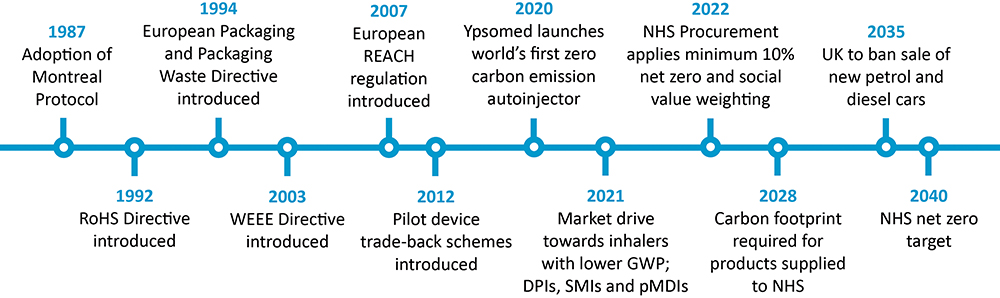
Figure 2: A timeline of sustainability progress since the adoption of the Montreal Protocol.
Development of a truly sustainable drug delivery device is a complex challenge. However, with each sustainable innovation, the sector will move closer to responsible development of medical devices to benefit both people and the planet.

