Citation: Fluetsch G, Chung L, “Expanding Automation Capacity for High and Low Volumes”. ONdrugDelivery Magazine, Issue 99 (Aug 2019), pp 6-9.
Gilbert Fluetsch and Lucy Chung look at how automation and assembly services can best evolve and adapt to industry standards and market demands, whilst delivering consistently at high quality and in a timely manner.
With the technological advancement of autoinjectors, the increasing development of biologics and the growing popularity of home-care self-injection, the demand for autoinjectors continues to flourish.1 Pharma companies are now increasingly in a race to launch their products to market, and suppliers need to align with pharma’s development speed, delivering rapidly whilst maintaining high quality standards.
Having been one of the major players in the drug device industry for 30 years, SHL knows all about the shifting demands in the autoinjector markets. Initially, SHL’s manual and semi-automated manufacturing capabilities were sufficient to fulfil low- to medium-volume device orders that covered just a few therapeutic areas. These capabilities also provided customers with a level of flexibility for clinical or commercial needs. Today, autoinjectors have become one of the more prevalent solutions for self-treatment of biologics, and both the range and number of demands are increasing.
“Collaboration between machine builders and device designers in the initial stage of device development could significantly increase productivity since designers have first-hand knowledge of designing components most suitable for automation assembly.”
When multiple customer demands require a production output of millions of devices per year, there is an increasing need to scale-up manufacturing production. Consistent quality that meets the highest regulatory requirements also needs to be addressed when scaling-up. Automated assembly machinery offers the reliability to produce high-volume productions with identical quality from the first assembled unit until the last unit. SHL has a strong knowledge base – from multiple electronic and software engineers and experienced project managers – when it comes to building automatic assembly machines for autoinjectors. Each machine system is fully researched and customised for multiple automation requirements.
To achieve the specified design requirements for the manufacturability of an autoinjector, SHL’s cross-functional teams of industrial designers, assembly system engineers, tooling engineers and moulding operations must collaborate closely to deliver satisfactory results. For example, the automation department must assess whether components are applicable in bowl feeders for assembly production, then collaborate with design teams to develop the precise specs of a component for automated assembly.
SHL’s strength in developing in-house automation lies in the parallel development process across numerous departments in one organisation. This means the design,tooling and automation phases all begin development at an early stage. For the automation department, collaboration between machine builders and device designers in the initial stage of device development could significantly increase productivity since designers have first-hand knowledge of designing components most suitable for automation assembly. Streamlined communication across departments increases production efficiency and eliminates potential risks that might occur during a linear process.
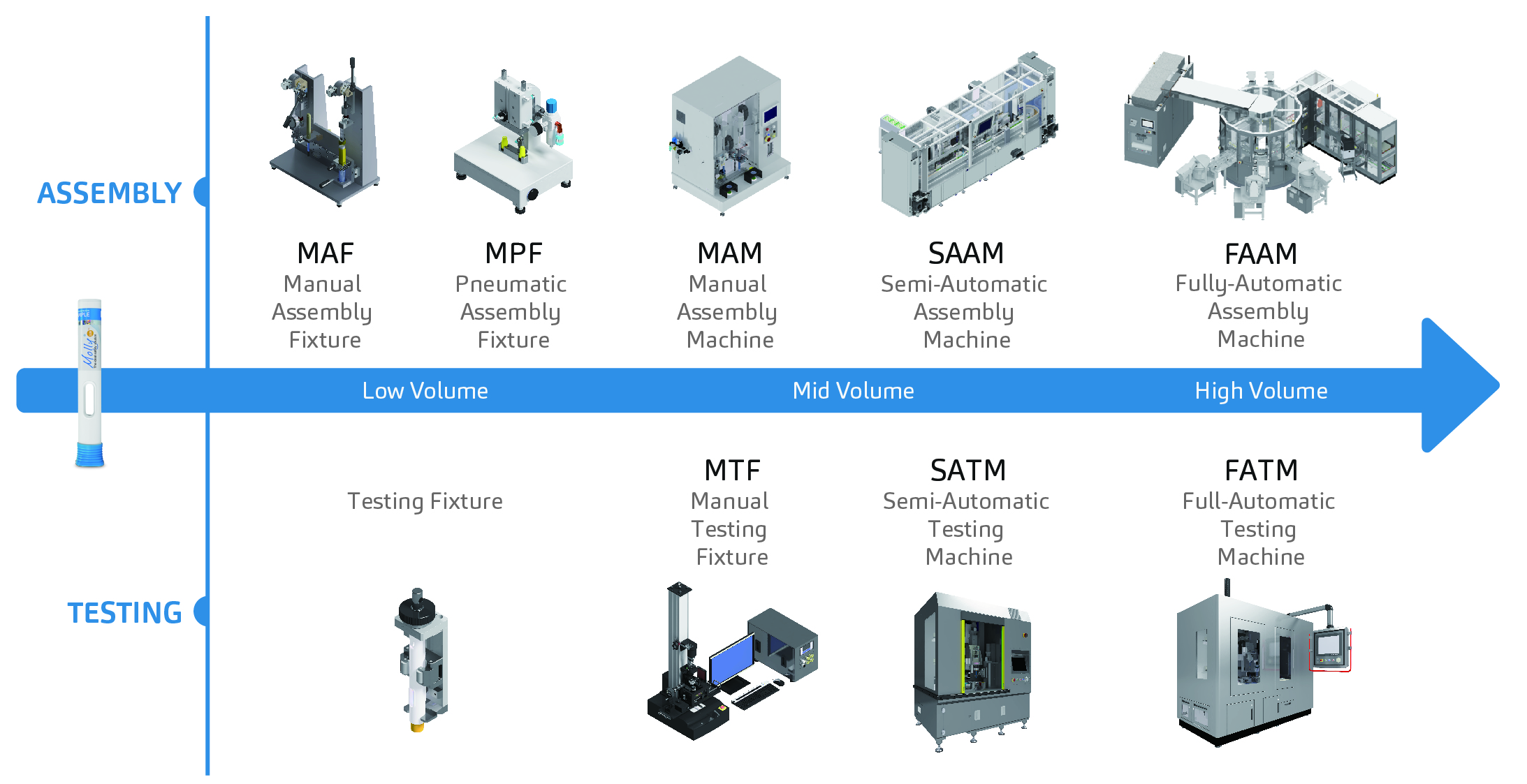
Figure 1: SHL’s automation system department machine offerings.
Failure modes and effects analysis (FMEA) and process failure mode effects analysis (PFMEA) carried out in close collaboration between the device design, quality, manufacturing and risk teams are also an important advantage in establishing an in-house automation department.
With the ability to design and manufacture assembly and testing equipment in-house, SHL’s automation department offers a wide range of manual, semi-automatic and fully automatic machine systems. Assembly requirements for manual and fully automated equipment vary greatly.
“A strong project management department ensures projects stay within the specified time frame and budget.”
In general, SHL’s automation department addresses two core capabilities – assembly and testing. Within these two main categories, SHL offers sub-assembly and final-assembly machine systems, as well as final device testing and sub-assembly testing services (Figure 1).
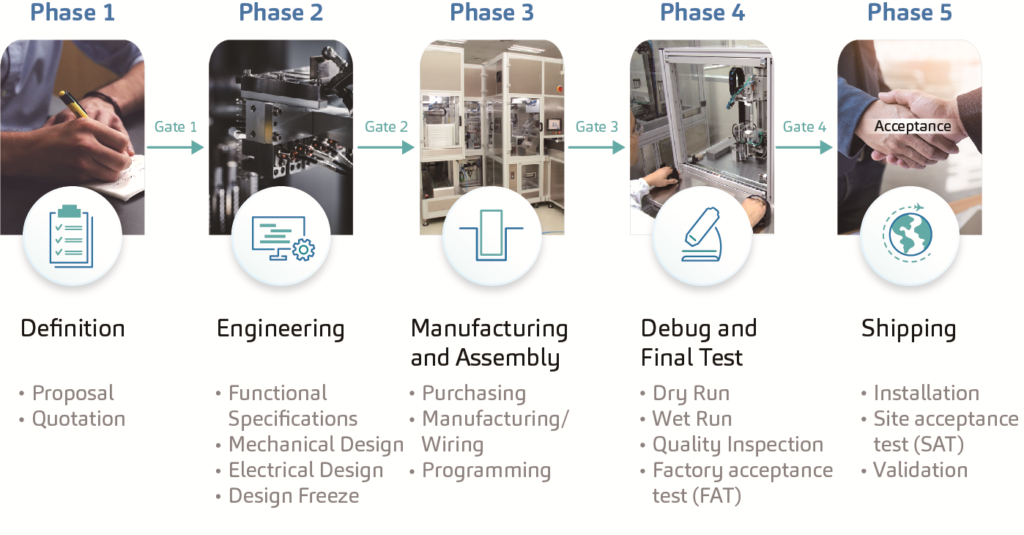
The design and development process of SHL’s automated equipment complies with a standard procedure which includes the definition phase, the engineering phase, the manufacturing and assembly phase, the debug and final test phase, and the shipping phase (Figure 2).
Regarded as the most critical step, the definition phase demands top-level clarification and a fully agreed set of specifications to ensure the successful flow of future operations. In other words, to alter a drawing or the module of a machine on a 3D CAD drawing is unquestionably more achievable than replacing or modifying parts on an actual machine. A strong project management department ensures projects stay within the specified time frame and budget. Mechanical and software engineers transform ideas into reality by following systemised procedures and guidelines that determine the accuracy of the developed equipment. These machines are developed in accordance with the provided designs and processes that comply with the requested GAMP5, ISO 9001 and CE standards.
Another important factor in the development process is software validation. Stringent validation processes are implemented to meet FDA 21 CFR Part 11 regulations. Furthermore, in-house service engineers provide on-site maintenance and services to SHL’s assembly and test equipment to ensure continued operations without issues in a 24/7 work environment.
THE MODULAR APPROACH
In an effort to produce high-quality and high-volume output, SHL introduced a modular design approach to the equipment base. With this, SHL is able to provide a standardised platform to build high-, medium- and low-volume output equipment. The modular design regarding manufacturing equipment also means a repeatable system that can be used in several production lines. This repeated method supports SHL’s goals to drive down costs, reduce risks, shorten lead times and increase production efficiency.
Modularisation in assembly allows for the standardisation of key manufacturing machinery and processes. The benefits include increased production efficiency, reduced costs and a faster output rate.
Designing a modular platform for multiple functions has its challenges. For instance, a final device consists of less than five parts that need to be assembled; in comparison, a sub-assembly consists of anywhere from four to 15 individual moulded or metal parts. The contrast in the number of parts between these two major units poses a challenge in designing an all-standard platform that supports both functions. Furthermore, the process and assembly requirements for a sub-assembly are quite different from a final assembly.
SHL owns the capacity to overcome such challenges and has designed multiple modular platforms that could serve as the base for both automated final assembly machinery as well as sub-assembly machinery.
INCREASED FLEXIBILITY
“Drug products experience numerous clinical trials,
pilot market testing, user feedback or even regulatory
issues that could well cause an impact on the
quantity of an order from a customer.”
Another example of a modular design approach is SHL’s multi-device equipment strategy, designed to assemble a higher mix of devices on the same equipment. This “low volume/high mix” idea also supports SHL’s goals to help customers reduce costs or other additional investments. For instance, building an assembly machine for low-volume batches would include additional design and development time for brand new equipment as well as unwanted idle time for the machine. The process can be compared with building a machine from scratch, having it operate for a few hours, and then having it sit around all day. With a modular platform designed with interchangeable fixtures and grippers, however, SHL has the ability to operate a variety of devices or several different versions of the same device on the same machine. This significantly reduces the equipment idle time, saving time and resources. That way, the machine can run 24 hours a day while supporting a diversified range of offerings.
The multi-device equipment strategy signifies SHL’s flexibility to meet several market demands, especially for customers with bespoke product specifications. Although the most perfect scenario would be to operate on modular platforms with high-volume production, the industry requires SHL to adapt and raise its standards of flexibility.
BEYOND HIGH-VOLUME DELIVERIES
The importance of offering a more flexible automation programme beyond high-volume production lies in the propensity of the rapidly changing medical device industry. Drug products experience numerous clinical trials, pilot market testing, user feedback or even regulatory issues that could well cause an impact on the quantity of an order from a customer.2 Some customers would opt for lower volume production to reduce risk and investment. Therefore, with regards to the varied market needs, raising the capacity for high-quantity developments would not always be the accurate response.
Contrary to high-volume production output with singular, dedicated processes without needing changeovers, low-volume production output requires several dedicated processes to support the flexibility in manufacturing. By interchanging several components on one singular machine – or strategically mixing and matching different module types – SHL ensures the reusability of a modular system for the vast and often varied demands from the client.
“Using modular platforms reduces the complexity of redesigning a new testing machine and further reduces the risks that might occur during the design process. The more streamlined an operation is – and the less manual input required – the fewer chances for mistakes to happen.”
This significantly expands its capabilities in providing a wide range of deliverables in different quantities, building confidence among customers with varied market needs.
AUTOMATING TESTING PROCESSES
With such stringent quality standards, SHL pays detailed attention to the testing of autoinjectors. Comprised of a primary container and 15–20 components of plastic and metal material, an autoinjector calls for several items to be tested to ensure safety for end users. Such is the complexity in the mechanism of an autoinjector, in particular when the moment of activation includes the sudden release of a spring force that drives the needle forward into the skin.
For device testing, SHL offers an integrated testing system that is applicable for both autoinjectors and pen injectors. The system’s configurability to the user’s preferences helps ensure the device meets its usability requirements, while detailed data analysis of multiple test items expands the scope for future product research and development. SHL also offers fully automatic testing equipment for mass-produced devices, with customisable options in terms of hardware and software for the convenience of the user. The equipment offers stable test parameters whilst supporting various test devices.
In response to high market demand, SHL has also approached the testing systems with modular designs, offering faster changeovers, ease of configuration and heightened flexibility. The importance of leveraging modular designs in testing equipment is significant in reducing lead times and risks. The test items of an autoinjector vary according to the device’s proprieties – with the number ranging from 10-20 items. Using modular platforms reduces the complexity of redesigning a new testing machine and further reduces the risk that might arise during the design process. The more streamlined an operation is – and the less manual input required – the fewer chances for mistakes to happen. In terms of reducing lead times, a modular platform eliminates the need for particular revalidation processes due to its redundancy. By altering a few component or equipment parts to accommodate test devices, the modular platform ensures a smoother process in testing procedures – significantly reducing the production timeline for customers and ensuring a speedy delivery for SHL. Moreover, the “low-volume/high-mix” strategy also applies to modular testing equipment, adding further value to the modular programme when lower-volume output is required.
Regarding SHL’s offering, the company’s automation and assembly services will continue to support the needs of its customers, by evolving and adapting to industry standards and market demands, while promising output of high quality and consistent, timely delivery.
REFERENCES
- “Global Autoinjectors Market 2019-2023”. Research Report, TechNavio, 2018.
- Schneider E, “Low-Volume Manufacturing”. Am Soc Mechanical Eng, December 22, 2010.