To Issue 182
Citation: Müller M, Müller S, “From Concept to Series Production – Accelerating Industrialisation in Medtech”. ONdrugDelivery, Issue 182 (Jan 2026), pp 64–69.
Matthias Müller and Sven Müller discuss Contexo and Probotec‘s combined approach to scaling drug-device combination products from laboratory prototypes to fully validated GMP commercial-scale production lines.
The journey from a laboratory concept to a fully validated, high-volume medical device production line has always been one of the biggest challenges faced by the drug delivery industry. The path is steep – prototypes must become reproducible products, pilot lines must evolve into stable serial production and every step must comply with strict regulatory frameworks, including GMP, ISO and the US FDA. For many manufacturers, this process takes far too much time – time that can decide whether a product reaches the market early or is overtaken by competitors.
In a sector where time-to-market is as critical as product quality, industrialisation speed has become a decisive competitive factor. However, acceleration cannot come at the cost of validation, especially in drug-device products or precision delivery systems, every process step – from component feeding to final packaging – must be proven, documented and repeatable under production conditions.
This is where the partnership between Contexo, a specialist in mechanical engineering and process automation (Figure 1), and Probotec, an expert in GMP-compliant production and operations, comes in. Together, these two companies have developed a seamless industrialisation approach that compresses the path from concept to series production – often by several months – without compromising regulatory compliance or technical depth.

Figure 1: Mechanical engineering and process automation.
THE INDUSTRIALISATION BOTTLENECK
Medical device developers often reach a critical point once prototypes have demonstrated functionality. At this stage, the focus shifts from product design to manufacturability, and the following questions need to be answered:
- Can the process be automated without sacrificing flexibility?
- How can validation be achieved quickly and efficiently?
- How can production be scaled once market demand increases?
“CONTEXO AND PROBOTEC CHALLENGE THIS PARADIGM WITH AN INTEGRATED, CONCURRENT APPROACH – INSTEAD OF ISOLATED PROJECT STAGES, THEY DEVELOP THE MACHINE, PROCESS AND PRODUCTION ENVIRONMENT IN PARALLEL.”
Traditionally, these phases have been sequential; first comes machine design, then process definition and finally the setup of the production environment. Every interface, between design and production and between automation and operation, introduces potential delays, misunderstandings and risks.
Contexo and Probotec challenge this paradigm with an integrated, concurrent approach – instead of isolated project stages, they develop the machine, process and production environment in parallel. The result is a validated, fully documented production system ready for operation within months.
TIME-TO-MARKET MEETS VALIDATION: THE CONTEXO & PROBOTEC MODEL
Customers no longer need PowerPoint concepts; they need functioning production systems. By synchronising engineering, validation and operation, Contexo and Probotec can eliminate waiting times and interface losses (Figure 2). At the core of the companies’ collaboration lies the idea of “One Flow”, wherein machine and process design, qualification and production start-up are all treated as a single, tightly integrated workflow.
Figure 2: From product idea to fully qualified production system.
END-TO-END PROCESS DESIGN
Contexo develops complete process architectures, from feeding to palletising. Every detail – part handling, sorting, dosing, laser welding, ultrasonic sealing, vision inspection, traceability and final packaging – is simulated, verified and documented (Figure 3). Meanwhile, in parallel, Probotec establishes the production environment, from layout design to infrastructure and cleanroom qualification, up to full GMP certification.

Figure 3: Contexo and Probotec develop all necessary process steps.
SIMULTANEOUS BUILD-UP AND TESTING
While machines are being built and tested at Contexo, Probotec prepares the production site, including logistics and operational quality systems. This parallel validation concept enables joint factory and site acceptance tests (FAT/SAT) under real production conditions. The outcome is a verified and audit-ready production line that meets all technical and regulatory requirements from day one.
RAPID RAMP-UP AND CONTINUOUS IMPROVEMENT
Once production starts, the joint team monitors efficiency, yield and quality in real time. Inline monitoring, statistical process control (SPC) and predictive maintenance are integrated from the outset. Adjustments and process optimisations occur without downtime, forming a continuous improvement loop. The result is that products reach the market earlier, in higher volumes and with consistent quality – all under full GMP documentation.
“CONTEXO AND PROBOTEC OFTEN START WITH A BASIC CONCEPT AND A PRODUCT IDEA AND, AFTER ABOUT EIGHT MONTHS, THE CUSTOMER LEAVES WITH A FULLY QUALIFIED PRODUCTION SYSTEM, COMPLETE DOCUMENTATION AND AN OPERATIONAL PRODUCTION LINE.”
BRIDGING THE GAP BETWEEN DEVELOPMENT AND PRODUCTION
Many medtech companies approach industrialisation with incomplete specifications. Contexo and Probotec often start with a basic concept and a product idea and, after about eight months, the customer leaves with a fully qualified production system, complete documentation and an operational production line. This is possible because the two companies cover the full value chain – from engineering to operation:
- Contexo – Machinery: Feeding, sorting, handling, dosing, welding, crimping, assembly, pressing, vision-inspection, laser marking, packaging concepts and palletising
- Probotec – Operations: Material flow, logistics, traceability, process operation, quality control, inline monitoring, SPC, data analytics, storage, dispatch and documentation.
The combination of the two creates a direct transfer of know-how with no media breaks, no interpretation gaps and no redundant validation cycles. Each process step is verified in the exact environment that it will later operate in. This eliminates the common scale-up surprises that often occur when moving from laboratory automation to industrial throughput.
MODULARITY ENABLES SPEED AND FLEXIBILITY
For medical device and combination product manufacturers, flexibility is key. Market demands and design changes can occur even during late-stage development. Contexo’s modular machine platforms are designed to absorb such changes without major redesigns. Whether a customer needs half a million or 200 million units per year, Contexo can scale the system accordingly, as the company’s mechanical and software architecture enables quick expansion while maintaining validated performance.
SCALING UP WITHOUT LOSING CONTROL
Once products enter the market, the challenge shifts to scaling production. Here, the Contexo and Probotec model ensures that growth does not compromise compliance or cost efficiency. Probotec takes responsibility for line operation, preventive maintenance and process monitoring. Data are collected from every machine and analysed in real time, enabling predictive maintenance and continuous yield optimisation. Simultaneously, Contexo provides the engineering backbone for technical upgrades and capacity expansion. Both partners ensure that every change, update or extension remains fully documented and validated.

Figure 4: Integrated supply chain and logistics.
INTEGRATED SUPPLY CHAIN AND LOGISTICS
Beyond machinery and process control, Probotec integrates procurement, warehousing and material flow management (Figure 4). This holistic approach ensures that the production line not only operates efficiently but also stays supplied and compliant throughout the entire product lifecycle.
The packaging and dispatch process is likewise automated – from unit labelling and serialisation to palletising and shipping documentation – everything is designed to meet international traceability standards.
VALIDATION FROM THE START – NOT AS AN AFTERTHOUGHT
One of the major advantages of the Contexo and Probotec approach lies in its validation philosophy.
Rather than treating qualification and documentation as separate project stages, validation is built into every step of development. Each component, process parameter and test sequence is logged and traceable from the first prototype.
This design-for-validation mindset ensures audit readiness at every milestone. The system documentation is generated automatically, covering GMP, hazard analysis and critical control point (HACCP) and ISO requirements. When authorities or notified bodies audit the production line, every record is consistent, verifiable and electronically archived. This approach saves weeks of preparation during regulatory submissions and supports faster approval processes – a crucial factor for drug-device manufacturers entering multiple global markets simultaneously.
“INDUSTRIALISATION IN MEDTECH IS NOT ONLY ABOUT COMPLIANCE – IT IS ALSO ABOUT ECONOMIC RELIABILITY.”
COST AND PERFORMANCE GUARANTEES
Industrialisation in medtech is not only about compliance – it is also about economic reliability. Both Contexo and Probotec offer performance guarantees:
- Cost Guarantee: Stable production costs through standardised, optimised processes
- Availability Guarantee: Defined output and uptime levels secured by predictive maintenance and spare-part management.
These contractual guarantees provide transparency and planning security, which is especially valuable for start-ups and scale-ups with investor-backed milestones.
INTELLIGENT AI AUTOMATION
Contexo has taken a decisive step in its digital evolution with the integration of OperAID (LEAD Digitalisierung, Winterbach, Germany) – an artificial intelligence (AI) automation platform engineered to support the manufacturing of medical devices, diagnostic components and primary packaging systems (Figure 5). OperAID systematically captures, structures and automates process and machine knowledge across complex assembly and inspection systems. By applying advanced AI algorithms, the platform transforms raw machine and process data into actionable insights that support predictive maintenance, process optimisation and quality control.
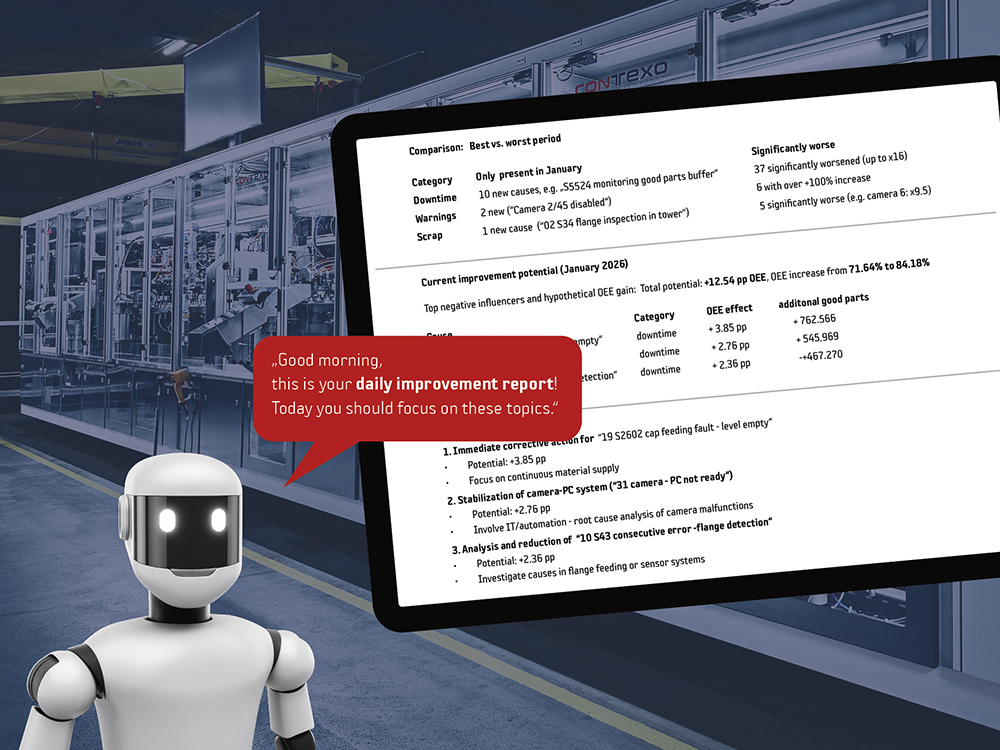
Figure 5: Intelligent AI automation to support the manufacturing process.
Through the integration of data from multiple sources, OperAID enables consistent information flow throughout production environments. This digital connectivity facilitates automated documentation, reduces manual intervention and strengthens traceability and compliance – critical requirements in regulated medical and pharmaceutical manufacturing.
FROM PILOT TO 24/7 PRODUCTION
In one recent project, a European medical device manufacturer sought to scale its drug delivery component production from a pilot line to full-scale, GMP-compliant serial manufacturing.
Within eight months, Contexo and Probotec delivered a fully validated system with:
- Simultaneous engineering and qualification of machines and cleanroom environments
- Integrated FAT/SAT with complete documentation package
- Production ramp-up to continuous operation
- Scale-up capability without the need for a layout redesign.
The client achieved market launch nearly half a year earlier than planned – a competitive advantage that translated directly into higher market share and earlier revenue generation.
COLLABORATION AS A SUCCESS FACTOR
What makes this model particularly effective is not only the technology but the organisational integration. Both companies work as one interdisciplinary team, sharing responsibility for process performance and regulatory compliance. This “One Team – One Flow – One Result” philosophy replaces traditional supplier-customer relationships with genuine partnership. Communication barriers disappear and projects progress with clear accountability and mutual transparency.
“THE ACCELERATION OF INDUSTRIALISATION PROCESSES IN MEDTECH IS PART OF A BROADER TREND TOWARDS ADAPTIVE MANUFACTURING – THE ABILITY TO MODIFY AND SCALE PRODUCTION QUICKLY WHILE REMAINING WITHIN VALIDATED BOUNDARIES.”
TOWARDS ADAPTIVE MANUFACTURING IN MEDTECH
The acceleration of industrialisation processes in medtech is part of a broader trend towards adaptive manufacturing – the ability to modify and scale production quickly while remaining within validated boundaries. With modular platforms and concurrent validation, the Contexo and Probotec approach aligns perfectly with this vision. It enables manufacturers to respond flexibly to design updates, new product variants or changing regulatory requirements, without restarting qualification from scratch. As medical devices become more complex, such agile, validation-oriented production concepts will be indispensable.
EIGHT MONTHS AHEAD
In an industry where every week counts, shortening the path from concept to series production by months is a decisive edge. By merging machine building, process automation and GMP-compliant operations into a unified development stream, Contexo and Probotec have redefined what industrialisation speed and validation can mean for the medtech sector. Faster market entry, validated quality from day one and scalable production without risk – this is the promise of their approach.

