Citation: Ash M, Lawrie-Fussey T, De Leeuw B, “How a Digital Approach can Unpick Clinical Trial Challenges and Improve Outcomes”. ONdrugDelivery Magazine, Issue 102 (Nov 2019), pp 14-16.
Matt Ash, Tom Lawrie-Fussey and Bastiaan De Leeuw look at the role digital technology can play in the development of drug-device combination products.
Clinical studies of drug and device combinations form a critical part of drug development programmes, yet significant uncertainties can be introduced from errors related to patient use. Billions of dollars are invested each year in clinical studies for new pharmaceutical products – either new molecular entities or reformulations of existing products to address additional indications. Yet for drug-device combinations in particular, if patients have difficulty effectively administering the drug, they may not receive the therapeutic benefit.
“The increased emphasis on human factors in the device regulatory pathway has given rise to enhanced levels of understanding of the interaction between patient usage and drug administration – but significant issues remain.”
Inhalers, for example, are notoriously difficult to use. The perception that “it seems to be a simple device to use” can mask underlying mismatches between the patient’s mental use model and the actual device operation – which can result in subtle errors that have a significant effect on drug performance.
Even in closely monitored studies, such as human factors usability studies, only a qualitative assessment of correct technique is possible. And in Phase III studies, which are often unsupervised, the only chance to explain any spurious or outlying trial result is what has been recorded on the case report forms. Consequently, it is common for a number of participants to be removed from clinical trials without truly understanding how the drug was administered – and whether issues have been caused by difficulties in drug delivery device use or lack of efficacy of the drug product.
Currently within the capsule-based dry power inhaler (cDPI) space, many (early-stage) clinical study results are marred by the presence of anomalous data points. These issues, which arise because of, for example, use-related error and/or delivery variation – or at least the inefficient collection or lack of collection of data – ultimately obfuscate the true efficacy of the new drug-device combination.
A number of papers have set out the prevalence of use error across almost all inhalers, including DPIs. For capsule inhalers with similar user steps and modes of operation – such as the RS01, Aerolizer and HandiHaler – a range of specific failure modes has been identified. Some of the more significant of these are:
- Failure to insert and pierce the capsule (27% of COPD users, n=205 and 41% of asthma users, n=124)
- Failure to exhale away from the device prior to inhalation (73.2% of COPD users and 71.8% of asthma users)
- Failure to follow the correct inhalation profile (45.4% of COPD users and 53.8% of asthma users)
- Failure to hold the breath following inhalation (19.5% of COPD users and 33.3% of asthma users).1-3
The increased emphasis on human factors in the device regulatory pathway has given rise to enhanced levels of understanding of the interaction between patient usage and drug administration – but significant issues remain. However, new digital tools for data capture can potentially shine a light on remaining errors and provide a new opportunity to increase performance to new heights. Digital systems not only help us understand the performance of basic devices per se but – when used through the whole device development process – they can deliver better therapy and clinical outcomes.
Regulators such as the US FDA are encouraging the use of new technology to help patients use devices more effectively – for example, by providing better training and monitoring adherence. There are also opportunities to monitor supply chain performance to ensure quality and efficacy. Taken together, these innovations can certainly benefit the healthcare industry as a whole.
Connected drug delivery devices using Bluetooth Low Energy (BLE) to communicate with apps are becoming common digital solutions. But although this approach addresses some opportunities, a more holistic approach is needed to fully exploit the opportunities that digital connectivity can provide. If the “digital journey” starts by using technology to understand the patient experience better, it can then be focused on monitoring clinical trials before being refined again in the final device. This approach has the benefit of ensuring that only the most effective digital enhancements find their way into the final product, whilst the clinical trials are supported with a new level of digital monitoring.
“New digital tools for
data capture can potentially shine a light on remaining errors and provide a new opportunity to increase performance
to new heights.”
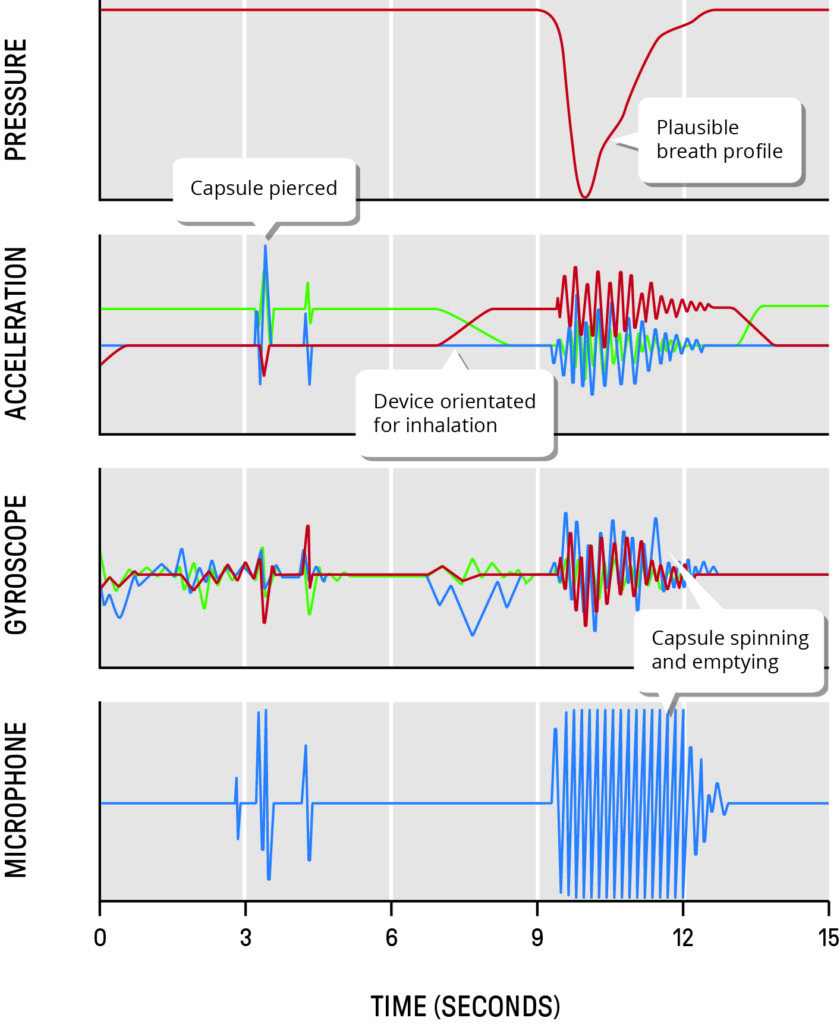
Figure 1: Readings from four different sensor types from DPI capsule loading through to completion of inhalation.
This opportunity involves adapting the latest, ever-growing suite of miniature low-cost sensors and device connectivity options that have been developed for the wearable devices industry, to gain a more detailed understanding of actual patient use of devices. Such quantified user insights provide an invaluable additional perspective over conventional human factors approaches.
Simply adding an array of the latest sensors is not enough. Usually more is gained from a data fusion approach. By equipping the device with a carefully chosen combination of sensors – probably more than would ever be included in a final commercial product – it is possible to create a much more accurate and robust assessment of actual patient use. For example, Figure 1 shows readings from four different sensor types through the complete patient experience of a DPI, from capsule loading through to completion of inhalation. Whilst each sensor alone can provide some insight, when combined in a time-synchronised, multi-vector suite, the overall fidelity of the system is greatly enhanced.
With suitably equipped devices, formative user studies can be used to assess potential devices and make minor modifications to optimise performance within time and budget constraints.
Once a well-informed device choice is made and the key points of weakness are identified, a subset of sensors can be carried over into clinical trials to identify those patient datasets where it is clear they were unable to self-administer the drug successfully. Although these sensing techniques do not solve the issues of patients who are unable to use the device, theapproach does enable a more informed conclusion with regards to drug efficacy, where patient outcomes can be linked back to successful administration procedures.
It’s important to remember, though,that in trials the stakes are high. The instrumented devices must, of course, be reliable and behave exactly like the native device, such that they don’t introduce new and unforeseen errors in patient interaction, technique or device misfunction – and regulators will need proof that this is the case. Creating a technology solution that captures and measures the complete sequence of events enables a far more granular and detailed picture of patient usage to be analysed. Also, by using a multitude of different sensor types, the conclusions drawn from a trial can be shown to be robust.
By this stage in development, the digital approach will have facilitated a much more detailed understanding of how patients interact with and use a device and how this influences drug performance. This creates a clear view of the value of digital aids in the final device offer, be they for training, monitoring or avoiding user errors.
A development programme that starts with comprehensive monitoring of formative testing enables a better understanding of patient device interaction and ensures that costly clinical trials are more informed and can be better designed. A subset of sensors can then be used to monitor the clinical trials devices to build additional knowledge and to ensure the data collected is due to the drug effect and not impaired by poor patient compliance or administration.
A small number of sensors may remain in the final device that goes to market. These will then have proven value in assisting the patient and the healthcare team to deliver the most effective therapy possible – maximising the value of a new drug launch and ensuring new lifesaving therapies make it into the hand of patients who need them.
REFERENCES
- Melani A, et al, “Inhaler mishandling remains common in real life and is associated with reduced disease control”. Respir Med, 2011, Vol 105(6), pp 930–938.
- Ocakli B, et al, “A comparative analysis of errors in inhaler technique among COPD versus asthma patients”. Int J Chron Obstruct Pulmon Dis, 2018, Vol 13, pp 2941–2947.
- Price D, Bosnic-Anticevich S, Briggs A, Chrystyn H, Rand C, Scheuch G, Bousquet J, “Inhaler competence in asthma: Common errors, barriers to use and recommended solutions”. Respir Med, Vol 107(1), 2013, pp 37–46.

