To Issue 150
Citation: Haddadi Y, Soukiassian H, “RFID-Based Unit-Level Traceability: Could it be the Key to Operational Excellence for Fill-Finish Lines?” ONdrugDelivery, Issue 150 (Jul 2023), pp 40–44.
Yacine Haddadi and Herve Soukiassian look at how BD’s track & trace model can help manufacturers improve their fill-finish operations.
Fill-finish operations are the final, critical phase before distribution of a pharmaceutical product. With its radio frequency identification (RFID)-based unit-level tracking solution for prefilled syringes (PFSs), Becton Dickinson is positioned to help pharma manufacturers address key risks and costly bottlenecks related to the fill-finish process. The BD solution for track & trace1 (T&T) has now been derisked through evaluation by a major pharmaceutical company, confirming several use cases, and is now ready to start large-scale implementation. Key use cases include the mitigation of mix-up risks, automation of the reconciliation process and elimination of over-segregation.
“Pharma manufacturers need to adapt their approach to risk mitigation in the face of new demands on modern fill-finish operations.”
TIMES ARE CHANGING – FILL-FINISH OPERATIONS NEED TO KEEP UP
With the industry adopting a platform approach to drug products, and with more products and dosages being filled in the same type of container on the same fill-finish lines,2 the risk of product mix-up is evolving, challenging current processes and manufacturing flows. The colour-coding systems typically used to prevent drug product mix-ups are reaching their functional limits. Today, pharma manufacturers need to adapt their approach to risk mitigation in the face of new demands on modern fill-finish operations.
Regulatory guidelines, existing and emerging, are also pressuring pharma to adapt fill-finish operations. To address quality problems that impact product availability and lead to product shortages,3 regulators are calling for more visibility of the manufacturing process,4 while also applying new scrutiny to how manufacturers ensure the integrity, quality and identity of their drugs. Examples include US FDA regulation 21 CFR610.14 that states that the manufacturer must ensure product identity after labelling, and USP Chapter <1790> concerning visual inspection, which requires manufacturers to determine the normal reject rate and investigate root causes.5 Furthermore, with the new Quality Management Maturity approach, the FDA is looking to reward companies that go beyond the cGMPs and implement solutions to ensure that “every dose is safe and effective and free of contamination and defects”.
The EU EMA is following the same rationale and has also initiated a working document to explore how digital innovations in manufacturing can help address the vulnerabilities of the medicine supply chain.6 Looking beyond the manufacturing site, end-to-end dose-level drug traceability is emerging as a likely next step of drug serialisation requirements.
Manufacturers also face pressure to align their fill-finish operations with new digital technologies as they seek to increase quality, lower the cost of goods manufactured (COGM), reduce batch release lead times and improve overall equipment effectiveness.7 According to a January 2023 report by McKinsey,8 the manufacture of sterile pharmaceutical products is set to grow by more than 50% over the next seven years, driven by new demand for recombinant antibodies and small molecules initiated during the covid-19 pandemic. Because of the time required to create and qualify new sterile manufacturing lines, pharma manufacturers must be prepared to tap the unused capacity of their existing lines. Attaining the additional yields necessary to respond to market growth will require new levels of operational excellence on fill-finish lines – something that is challenging without process automation and digitalisation.
SLAY SILOS WITH A HOLISTIC APPROACH
Risk mitigation, regulatory alignment and yield optimisation are often addressed as separate challenges, each requiring a different set of solutions. However, when examined carefully, these challenges are actually interconnected in fill-finish operations. Asset productivity is constrained by regulatory requirements that intend to mitigate the risk of releasing flawed products to market (Figure 1). The ability to track each product individually from the time of filling to its packaging can reduce this risk. And, by extension, doing so can unlock the potential for productivity gains by simplifying some of the process requirements currently restricting overall equipment efficiency (OEE).
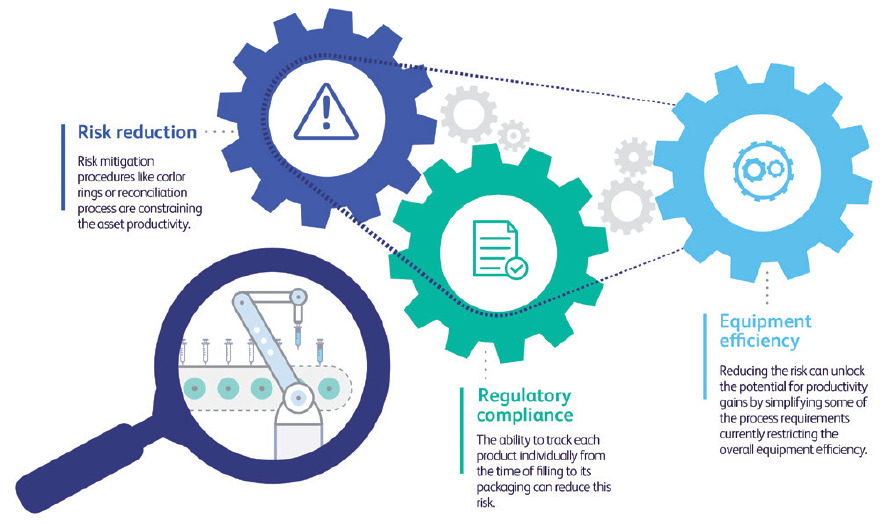
Figure 1: Interconnection between risk reduction, compliance and equipment efficiency.
“The BD solution for T&T consists of RFID-tagged syringes, tagged nests and cloud-based sharing of aggregation data.”
These challenges could all be addressed by the unit-level identification of primary containers. This would help pharma manufacturers build trust in their fill-finish processes by providing unit-by-unit accountability for each and every container on the filling line – and beyond. It would support manufacturers in addressing the barriers to quality and productivity inherent in many fill-finish processes, but which have not, until recently, been the focus of industry scrutiny. Unit-level traceability and precision would be at the heart of more efficient deviation management and reduce batch-level complexities to the clarity of a “batch of one” – replacing guesswork, inaccuracies and labour-intensive investigations with precise, continually updated unit-level data that can help resolve issues before they become subjects for investigation. This would also position manufacturers to build end-to-end value chains leveraging unit-level traceability from glass manufacturing to point-of-care (POC) applications.
SOLUTION OVERVIEW
The BD solution for T&T (Figure 2) consists of RFID-tagged syringes (the tag is located in the rigid needle shield (RNS)), tagged nests and cloud-based sharing of aggregation data (syringe-nest aggregation). Each RFID tag will incorporate a container unique identifier (CUID). At the end of the BD manufacturing process, tagged syringes are sealed into tubs and their CUIDs are read and aggregated to the nest ID. This aggregation of parent and child units is intended to facilitate reading at filling line entry where only the nest ID will be read, with the child CUIDs easily retrieved from the aggregation data. Each CUID will be associated with the current drug filling batch and drug code. The unit’s status at each step (i.e. successfully filled, rejected, etc) can be recorded and,at each subsequent process step, the syringe ID can be cross-checked to prevent mix-ups, even after assembly.
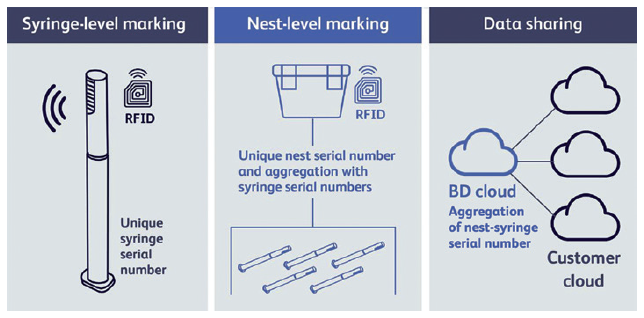
Figure 2: The BD solution for T&T.
Additional manufacturing events can be recorded to build a full syringe pedigree. Prior to release, the drug’s pedigree can be confirmed based on its movement through its pharma manufacturing steps. Additionally, the nest RFID tag will activate mass-reading use cases (e.g. read in pallets) thanks to its longer reading range.
“RFID technology represents several chief advantages over optical marking.”
The solution aims to offer throughput speeds of up to 1,000 units per minute, ensuring compatibility with the fastest fill-finish process speeds.9 As the solution uses industry-standard components,* pharma customers can choose their preferred partners for integration with their existing equipment and systems.
CHOOSE RFID TECHNOLOGY WHEN AGILITY COUNTS
RFID technology represents several chief advantages over optical marking. Because RFID readers require no line of sight, their placement is simple and flexible and can be installed outside of isolators. In addition, there is no need to rotate syringes, ensuring compatibility with existing machines and helping to maintain high throughput speeds while eliminating extra process steps and potential sources of error. The RFID tag can be read through autoinjectors, ensuring full visibility across all manufacturing steps.
Unlike optical marking, the RFID T&T solution requires no modification of the syringe barrel, which could potentially interfere with human- or machine-based visual inspection and detection of visible particles. The RFID unit is incorporated into the RNS without altering the RNS’s dimensions, maintaining compatibility with secondary devices such as autoinjectors and safety devices.10 In addition, the BD solution enables mass reading of units within nests and through secondary devices, cartons and pallets, for instantaneous identification of units at each lifecycle stage.
At the end of the manufacturing process, the syringe RFID tag could be re-encoded to carry drug information that can be used in a hospital setting to automate documentation, track recalls and improve inventory management.11 The solution has the potential to be extended to provide end-to-end traceability to meet the needs of connected ecosystem strategies including POC applications and the monitoring of patient outcomes.
Use Case 1: Addressing the Growing Risk of Mix-Ups12
The BD solution for T&T is intended to offer a robust alternative to the limitations of colour rings. Because it is based on individually identified containers with associated data, the T&T solution is designed to manage unlimited combinations of products/strengths, while colour rings are limited by the ability to distinguish many colours within manufacturing constraints (e.g. speed, light, etc). And because colour-coded rings are applied at the exit of visual inspection, a coverage gap exists when using them, allowing the possibility of mix-ups to occur between filling and inspection. However, with a single and continuous unit-level tracking system covering the entire fill-finish process and beyond, the T&T solution is intended to ensure that there are no gaps in vigilance against mix-ups. Additionally, removing the application of colour rings should streamline the process, supporting greater equipment efficiency.13
Use Case 2: Automating Reconciliation
The reconciliation step is intended to ensure that all materials have been accounted for at the end of a batch fill-finish process and that no mix-up occurred. It is a mandatory, labour-intensive step that must be completed before the start of a new filling batch. It requires counting incoming and outgoing units, and the final tally must account for 100% of the units, including rejects, samples and returns. Discrepancies must be investigated, and all units need to be accounted for to clear the line and commence changeover. Reconciliation is a significant pain point for many pharma manufacturing operations. Accounting for 100% of all materials used in a fill-finish process can be challenging and there is a risk of calculation error.
The T&T solution identifies and tracks every PFS unit as it moves through the fill-finish process. Any unit that does not make it out of a particular manufacturing step is identified during the process. Reconciliation issues can be resolved “on the fly”, making it possible to “blacklist” missing units, marking them for automatic ejection should they reappear in the process. Issues could be closed quickly, significantly accelerating the reconciliation process, leading to better OEE and faster batch release. With RFID-equipped unit-level PFS tracking, fill-finish operations could convert time previously lost during reconciliation into productivity.
Use Case 3: The End of Over-Segregation
Due to the lack of unit-level visibility of traditional fill-finish operations, when a quality issue occurs, segregating only affected units is challenging. Therefore, to ensure segregation of all affected products, manufacturers often over-segregate or even discard entire batches. The BD solution for T&T is designed to precisely link rejected units to each step of the process, making it possible to identify and segregate only those units affected by a potential quality issue. By eliminating the disposal of non-affected products, manufacturers could be able to optimise product delivery.
ADAPTING TO PRESENT AND EMERGING REGULATORY TRENDS
Pharma manufacturers face the challenge of implementing mature quality management systems that provide better visibility of the fill-finish process and which limit the number of deviations and investigations and, consequently, ensure that products will be released on time. In response to regulatory trends, manufacturers are expected to replace the traditional “detect and reject” approach to quality with proactive practices that enable continuous quality improvement and “right-first-time results”. This includes the ability to establish normal rejection rates, detect trends, determine root causes and implement corrective and preventive actions. The BD solution for T&T is intended to provide the process visibility central to reaching these goals.
CREATING VALUE THROUGH PHARMA 4.0
The International Society for Pharmaceutical Engineering defines Pharma 4.0 as “the era of smart machines, storage systems and production plants that can autonomously exchange information, trigger actions and control operations free of any human intervention”, conveying the notion of widespread digitalisation and automation throughout the industry. While companies may be at various levels of implementation of Pharma 4.0 strategies, the large majority are using data to monitor their processes, products and environments. However, as this data is not linked to the physical container, it is only used for a specific process step and cannot be leveraged to build a true end-to-end control strategy that encompasses the primary packaging, the pharma manufacturing steps and the possibility of going all the way to the patient.
Unit-level traceability represents a key digital enabler for a range of Pharma 4.0 applications, both within the manufacturing site and beyond it. The unique identifier can be used as the centrepiece of the data collection system, to bridge the organisation’s data silos connecting process variations with patient outcomes and enabling the next leap in drug product quality. Looking further, by providing pharma with access to container metadata (PFS length, flange dimensions, concentricity, etc) at the unit level, and by linking this to pharma manufacturing process data and, ultimately, to patient data, pharma manufacturers could be able to identify how small variations in the primary container or in their process can impact processability, quality and/or the patient experience. Ultimately, by moving scrutiny from the batch to the dose, a new level of trust in the process could be reached thanks to accountability at the unit level, paving the way for a “batch-of-one” approach, enabling real-time release of manufactured doses (Figure 3).
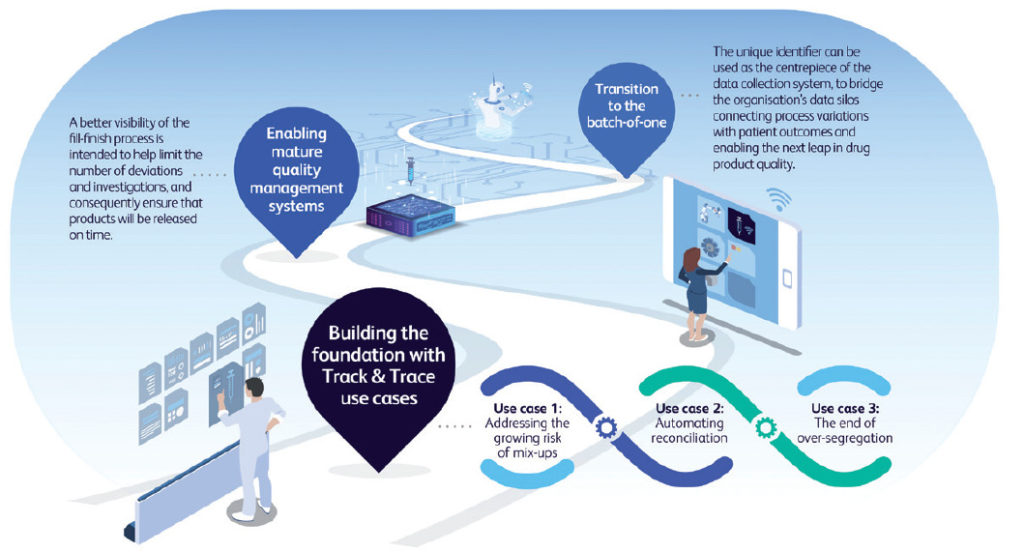
Figure 3: Unit-level identification is a foundation for Pharma 4.0 applications.
LOWER THE COST OF GOODS MANUFACTURED
BD’s RFID T&T solution is intended to support pharma manufacturers in lowering their COGM and increasing OEE. It is designed to address many of the sources of high fill-finish costs, helping pharma companies to:
- Reduce changeover downtime by accelerating line clearance and reconciliation
- Reduce the duration and complexity of investigations to ensure timely batch release
- Put a stop to over-segregation and full-batch discard, and reduce the overall scrap rate
- Mitigate exposure to mix-ups and minimise the risk of human error
- Increase manufacturing flexibility to better handle portfolio growth and pre
TOTAL COST OF OWNERSHIP: A STRONG BUSINESS CASE
BD has undertaken a comprehensive total cost of ownership analysis supporting a strong business case for its solution for T&T. The study can be made available to qualified partners who are evaluating the BD solution.
An Incremental Revolution
The BD solution for T&T can help pharma manufacturers bring new performance to their fill-finish operations in a way that is compatible with their operational and investment priorities. Scalable, flexible and future-proof, the T&T solution is intended to help pharma manufacturers adapt rapidly and cost effectively to the growing demands on their fill-finish operations.
BD invites all interested parties to reserve a visit to its demonstration lab for a proof-of-concept presentation of its RFID-based solution for T&T.
* UHF RFID tag, EPC™/RFID tagging protocol based on GS1 standards. By selecting no proprietary software or rules, GS1 standards enable any allowed supply chain participant across the globe to read data with proper RFID equipment.
REFERENCES
- “Policy paper on traceability of medical products”. WHO, March 18, 2021.
- Markarian J, “Automating Biopharma Manufacturing”, Pharm Technol. 2022, Vol 46(7), pp 30–33.
- “Quality Management Maturity: Essential for Stable U.S. Supply Chains of Quality Pharmaceuticals”. US FDA, Jul 2022.
- “Unique identification of primary containers to drive product traceability and quality”. ISPE, Feb 1, 2021.
- “Pharma Firm Explores RFID for Prefilled Syringes”. PDA Letter, Jun 23, 2020.
- “Commission staff working document – Vulnerabilities of the global supply chains of medicines – Structured Dialogue on the security of medicines supply”. European Commission, Oct 2022.
- Schrader U, “Operations can launch the next blockbuster in pharma”. McKinsey and Company. Feb 16, 2021.
- “How Sterile Pharma Manufacturers Can Grow Capacity without Capital Investment”. McKinsey and Company, 2023.
- “The current state of aseptic processing & fill-finish manufacturing”. CRB, Accessed Jan 12, 2023.
- Steffen Y, “RFID in RNS for 100% container traceability”. PDA Webinar, Feb 17, 2021.
- “RFID Uses, Benefits, and Costs: Literature Review and Key Informant Interviews”. The Axia Institute, Michigan State University, May 2022.
- “Supporting Becton Dickinson in assessing the opportunity for industrial traceability solutions”. Study conducted for Becton, Dickinson and Company, Feb 2020.
- “Primary research to assess the value creation for syringe identification”. Study conducted for Becton, Dickinson and Company, Jan 2022.

