To Issue 166
Citation: Masciopinto V, “SHL Medical’s Elexy™: Redefining Possibilities”. ONdrugDelivery, Issue 166 (Oct 2024), pp 78–82.
Vince Masciopinto introduces Elexy™ SHL Medical‘s new reusable electromechanical autoinjector, highlighting the various advantages that the device platform offers at preclinical, clinical and commercial stages due to its ability to deliver a variety of injection profiles from different primary containers without requiring any mechanical reconfiguration of the device.
As a leader in the self-injection space, SHL Medical has consistently been at the forefront of developments in the industry. In 2005, the company’s DAI® disposable autoinjector set the standard for the convenient use of modern autoinjectors. In 2010, SHL once again changed the industry by introducing Molly® as a device platform solution, enabling its partners to reduce investment, accelerate development and increase scale. With its latest product, Elexy™, SHL has developed a solution that could redefine what is possible for injectable drug delivery.
Elexy is a versatile, reusable electromechanical autoinjector that aims to provide a best-in-class user experience and the broadest available range of delivery volumes. Comprised of a reusable power unit and disposable drug cassettes, the Elexy system is compatible with both prefilled syringe (PFS) and cartridge primary containers and, capable of delivering up to 5 mL in a single injection (Figure 1). For cartridges, SHL has included its proven NIT® needle isolation technology, which removes the need for a user to attach a needle manually, providing a seamless injection experience.
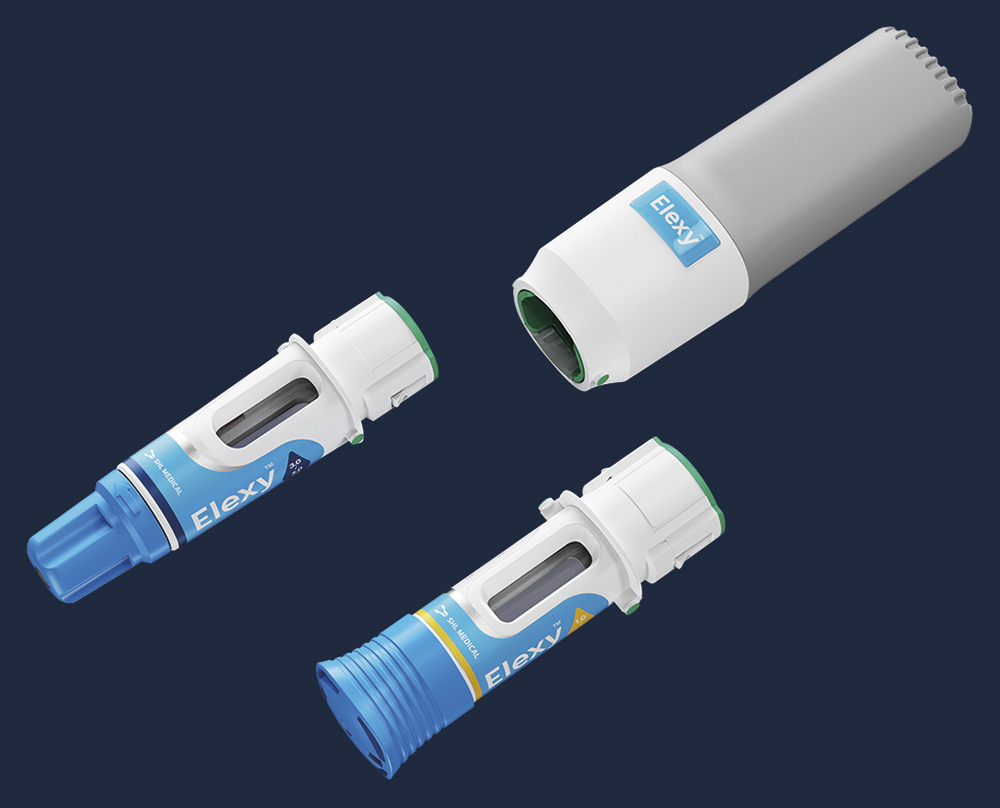
Figure 1: The Elexy autoinjector is comprised of a reusable power unit and disposable drug cassettes that can accommodate both PFS (Left) and cartridge (Right) primary containers.
“Performing an injection is identical to the procedure for SHL’s Molly single-use disposable autoinjector – simply remove the cap and push Elexy against the injection site to activate.”
The reusable power unit can provide a wide range of delivery rates and push highly viscous fluids, creating unprecedented flexibility without any changes to hardware. Performing an injection is identical to the procedure for SHL’s Molly single-use disposable autoinjector – simply remove the cap and push Elexy against the injection site to activate. Its multi-level feedback mechanism provides an enhanced user experience, including visual cues on the power unit and plunger, as well as audio and haptic feedback.
Elexy features integrated connectivity with cellular technology, opening avenues to collect rich injection data. The control of the power unit is provided by a radio frequency identification (RFID) tag on the disposable drug cassette, ensuring that the proper delivery parameters are used without the need for user inputs or creating opportunities for user error – it is as simple as loading a drug cassette.
“Innovative drug therapies are being developed that push the envelope of current delivery tools, requiring the delivery of larger volumes, higher viscosities and novel drug formulations.”
PRECLINICAL ELEXY: WHERE A COMMERCIAL TOOL MEETS DESIGN OF EXPERIMENTS
The history of parenteral drug delivery tends to repeat itself – from the development of the hypodermic needle to the PFS to the infusion pump to the autoinjector; as new therapies emerge, they require specialised tools to optimise delivery. Today, innovative drug therapies are being developed that push the envelope of current delivery tools, requiring the delivery of larger volumes, higher viscosities and novel drug formulations. Beyond the safety and efficacy of the drug-device combination product, it is also critical to consider patient tolerability, pain and preference to ensure commercial viability.
An in vivo preclinical study that varies delivery parameters, such as injection rate, delivered volume, injection depth and needle gauge, would be impractical or impossible to execute with a mechanical autoinjector. Elexy’s flexibility makes such a preclinical study to evaluate the injection performance and tolerability of delivering large volumes subcutaneously not only possible but as simple as loading a new drug cassette (Figure 2). Furthermore, the use of a hand-held autoinjector provides better, more representative data than other preclinical delivery options, such as a through a needle set applied with adhesive.1
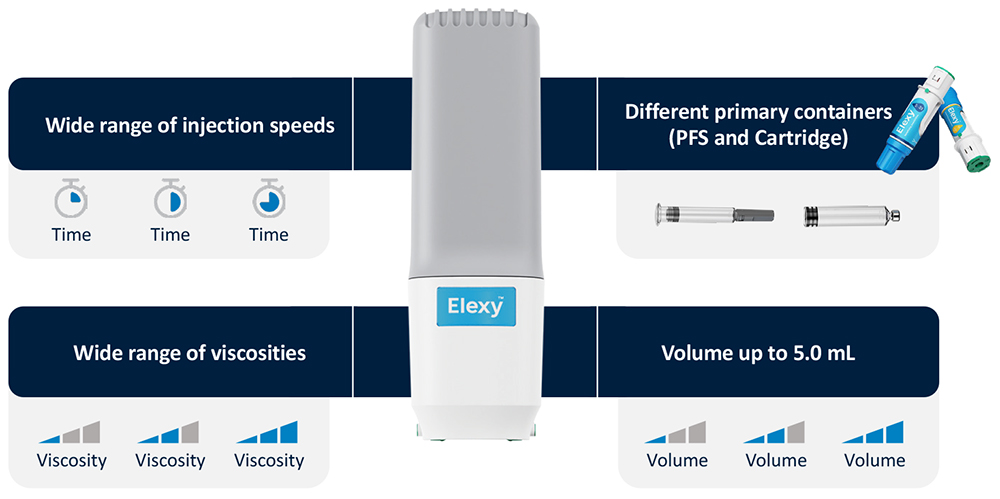
Figure 2: The architecture of Elexy allows for flexibility in injection parameters, making it possible to execute preclinical studies with a hand-held, self-injection device.
CLINICAL ELEXY: USING AN AUTOINJECTOR TO FIND THE RIGHT DOSE AND AVOID BRIDGING STUDIES
The typical approach to developing combination products is to focus on drug development first and worry about the delivery mechanism later. While this strategy has been successful in the past, it carries with it significant risk and uncertainty during the more expensive clinical and commercial phases and often requires bridging studies. These studies are costly, time-consuming and carry additional risks, sometimes delaying the commercial launch of the autoinjector.
An ideal approach to developing an autoinjector-based combination product would be to use an autoinjector during clinical trials and evaluate the combination product as a whole, avoiding device-related bridging studies altogether. This is impractical with single-use, mechanical autoinjectors because varying the dose requires significant component changes and long timelines to develop a new autoinjector for each test condition. On the other hand, Elexy uses the same power unit to deliver a variety of dose volumes, primary containers and delivery rates – making the execution of a dose-ranging study as simple as loading a new drug cassette for each use case. Additionally, Elexy’s flexibility with primary containers allows for easy transitions between low-dose, high-frequency regimens and high-dose, low-frequency formats, providing new options for clinical study design.
Using an autoinjector to generate clinical data, especially one with a programmable injection rate, produces a dataset that is much more representative of commercial applications. Additionally, because Elexy uses a standard primary container, the storage conditions and fluid path will also match the commercial application, resulting in shorter bridges and faster speed to clinic. Furthermore, the digital capabilities of Elexy enable the collection of richer clinical datasets and can instil greater confidence when transitioning to commercial supply. This provides two significant opportunities for acceleration through clinical studies – using Elexy as a commercial device with delivery identical to that used in the clinical trials or using the clinical outputs to better inform the commercial autoinjector design.
Single-use mechanical autoinjector design relies on certain critical information, specifically injection volume, injection rate, injection depth and formulation viscosity. The mechanical components must be modified or changed in some way to accommodate these inputs to optimise the delivery parameters. This development of the mechanical system takes time to implement, test, iterate on and validate.
“Using Elexy during clinical trials enables optimisation of delivery parameters alongside drug development, which can then be translated immediately into a mechanical autoinjector with very low risk.”
Due to the nature of clinical trials, these design inputs can only be known at the precise time that the product needs to advance to the next development phase or launch commercially. This results in delays or white spaces in development timelines. Attempting to circumvent this either leads to the use of suboptimal parameters during development to speed up timelines or launching commercially in a different device presentation, adding the autoinjector at a later date and driving up lifecycle management costs. Using Elexy during clinical trials enables optimisation of delivery parameters alongside drug development, which can then be translated immediately into a mechanical autoinjector with very low risk.
“Elexy’s electromechanical drive can easily deliver fluids where mechanical systems would struggle, such as with ultra-viscous fluids, profiled injections and those requiring precise delivery rates.”
COMMERCIAL ELEXY: FLEXIBILITY FOR DYNAMIC APPLICATIONS
Like other reusable delivery systems, Elexy offers significant advantages for chronic conditions or therapies that require multiple doses. The more the reusable components are used, the more these advantages are amplified. Furthermore, Elexy’s electromechanical drive can easily deliver fluids where mechanical systems would struggle, such as with ultra-viscous fluids, profiled injections and those requiring precise delivery rates. In cases where cartridge-based delivery is more favourable, the NIT-integrated drug cassette allows for complete control of the cannula gauge and length to support achieving the target injection time and depth, independent of the primary container.
What separates Elexy from other reusable electromechanical systems is its flexibility coupled with its usability (Figure 3). This is especially true for commercial applications where multiple doses are required or those that require different primary containers, such as variable doses (e.g. loading and maintenance doses) or multi-med therapies.
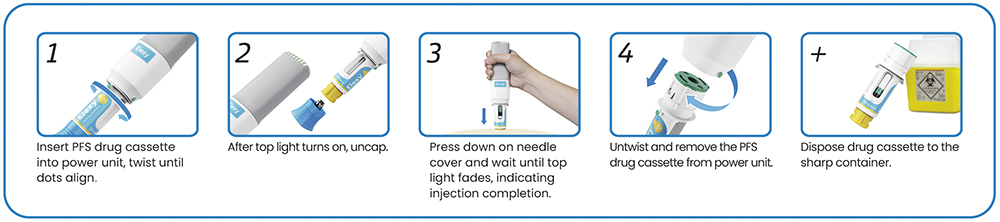
Figure 3: Reusability has minimal impact on Elexy’s usability. The device features a familiar two-step activation process with passive sharps protection (PFS drug cassette shown here as an example).
Elexy would be uniquely advantageous for a drug that requires a large loading dose and an ongoing smaller maintenance dose. A starter kit, containing a power unit and large-volume drug cassettes, could support initial loading doses. After completion of the starter kit, the patient could receive maintenance kits of multiple drug cassettes that would support the ongoing therapy. The user sequence would be identical for every injection, and the RFID in each drug cassette would ensure that the right delivery parameters are used without any intervention from the user. Beyond simplifying and optimising the injection experience for the patient, it would also provide significant advantages for package efficiency, storage, shipment, sustainability and error-proofing.
Elexy could also offer unique optionality for patients where dose is a choice or determined by factors expected to changeover time. The flexibility to deliver multiple volumes and use a variety of primary containers enables patients to use the same power unit with multiple drug cassettes. Without the need for user inputs, there is no risk of the wrong dose being delivered and the user experience is identical.
CONCLUSION
In the ever-changing landscape of parenteral drug delivery, new systems are required to address unmet needs – both for patients and in product development. Elexy is redefining possibilities as a flexible autoinjector capable of evaluating new therapies in accelerating, improving and de-risking clinical trials, as well as delivering dynamic doses to patients in new ways. The possibilities are broader than ever before with Elexy – it is as simple as loading a new drug cassette.
REFERENCE
- Dang X et al, “Clinical Investigation of Large Volume Subcutaneous Delivery up to 25 mL for Lean and Non-Lean Subjects”. Pharm Res, 2024, Vol 41(4), pp 751–763.

