To Issue 150
Citation: Arts H, Den Hollander J-W, Cisliek R “Streamlining Drug Delivery Device Development for Industrialisation Success.” ONdrugDelivery, Issue 150 (Jul 2023), pp 31–34.
Hans Arts, Jan-Willem Den Hollander and Ron Cisliek discuss the value of bringing an industrialisation partner on board early in product development and how working with a single manufacturing specialist can preserve insights and learnings throughout the product development lifecycle and deliver additional value and quality.
FROM PROTOTYPE TO PRODUCTION
The core challenge in the production of drug delivery devices is how to combine exceptional quality with cost-effective manufacturing. It is critical to guarantee the flawless operation of every single device, potentially up into billions of units, while also optimising costs to ensure affordability.
Industrialisation is key in taking on this challenge. This article discusses how each of the four development phases of a product mould can be optimised for industrialisation, advocating for a forward-thinking approach, where proactive and strategic investments into design and engineering lay the foundation for successful industrialisation. As part of this approach, considerations for industrialisation should be taken into account from the earliest stages of product development.
THE FOUR PHASES OF AN INJECTION MOULD FOR DRUG DELIVERY DEVICE
Before exploring how to optimise product development for industrialisation, it is important to understand the phases of product development. While different approaches are possible, the development of most devices consists of four main phases.
Phase 1: Proto-tools
The goal in the first phase of development is to create a concept that proves the device’s functional viability. To keep costs low, the product design is iterated upon with 3D printed device samples and single-use silicone moulds. At the conclusion of this phase, a proto-tool is produced. This is typically a “soft mould” made from aluminium or non-hardened steel (Figure 1).
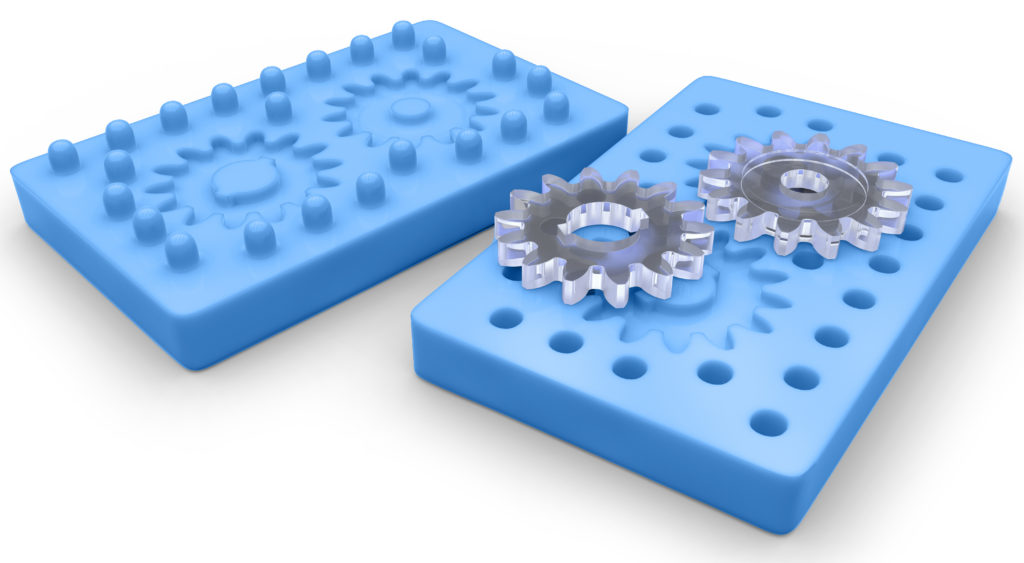
Figure 1: 3D-printed silicon mould.
Phase 2: Submission Tool
To ready the device for submission to the US FDA or EMA, “pre-production” moulds are required. These moulds are made out of hardened steel and tend to have between one and four cavities. These submission tools are suitable to produce the first few millions of devices, after which they often serve as back-ups.
Phase 3: Launch
After approval, the new drug product is put on the market. After the long approval and testing process, there is a high degree of confidence that the product will be successful. For this reason, original equipment manufacturers (OEMs) tend to opt for multi-cavity moulds in order to have sufficient production capacity (Figure 2).
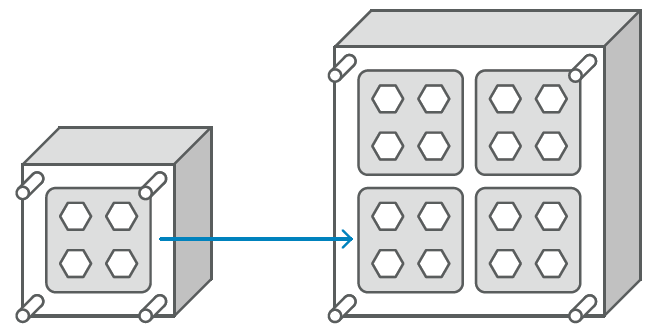
Figure 2: From submission tool to multi-cavity moulds.
Phase 4: Ramp Up
Once the drug has proved successful, it is time to scale up the production capacity. At this point, OEMs can either decide to use repeat moulds or moulds with a higher number of cavities.
“As a high-precision mould maker, IGS GeboJagema has shown many times how cost effective industrialisation starts in the earliest stages of product development.”
OPTIMISING EACH DEVELOPMENT PHASE FOR INDUSTRIALISATION
At first glance, it might seem like industrialisation becomes most relevant only after the drug product is approved, in Phases 3 and 4. However, as a high-precision mould maker, IGS GeboJagema has shown many times how cost-effective industrialisation starts in the earliest stages of product development.
During Phase 1, the primary objective is to design a product that is both easy to use for patients and effective in delivering the drug. IGS GeboJagema supports its customers in this phase by sharing its design for manufacturing (DFM) expertise. This is where the IGS team optimises the product design for efficient production further down the line. For example, seemingly small product design changes can reduce cycle time or significantly lower mould costs – adjustments in this early stage can have a significant impact on a project’s bottom line (Figure 3).
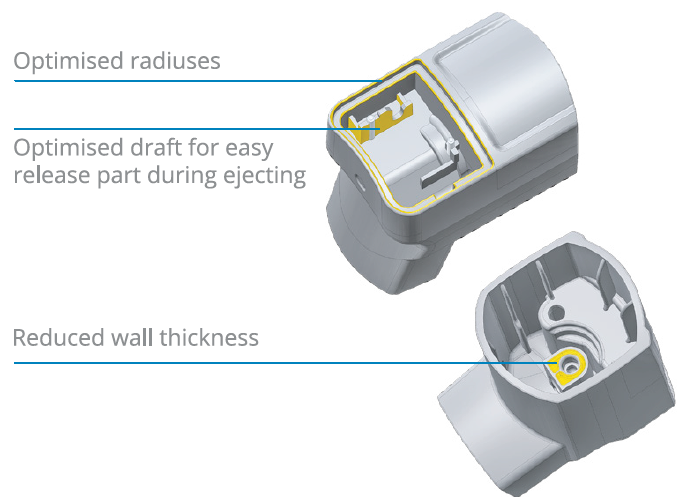
Figure 3: Small changes in the product design can simplify the manufacturing.
As a rule of thumb, it is advisable to involve industrialisation-focused partners for 75% of the first product design. Furthermore, IGS GeboJagema works together with automation companies to ensure that the product design is suitable for efficient assembly, which is known as “design for assembly”. More information about DFM can be found in IGS GeboJagema’s white paper on the topic.
“While there will be some learnings from the development of the single-cavity mould, the larger cavity stack will introduce new challenges. In practice, this means that much of the work is done twice.”
As described, in Phase 2 OEMs usually opt for a mould with no more than four cavities. The advantage of this is that it saves costs and time at this relatively early stage of the product development. However, the drawback is that it requires designing and programming a new, multi-cavity mould when the product is ready to be launched in Phase 3. While there will be some learnings from the development of the single-cavity mould, the larger cavity stack will introduce new challenges. In practice, this means that much of the work is done twice.
For this reason, when OEMs are confident that their drug will be approved, IGS GeboJagema often suggests a different approach. Instead of engineering a single- or low cavity mould, the company designs and programs a 16- or 32-cavity tool(Figure 4). This involves almost the same amount of work as developing a single cavity mould. After completing that mould engineering concept, it is fairly straightforward to take the cavity stack and manufacture a smaller injection mould for it. This approach is an investment that is highly beneficial during the launch of the product in Phase 3, allowing the client to take advantage of 100% of the learnings of the pre-production tool. Moreover, the submission cavity stacks can be reused in the multi-cavity mould or be kept as a spare.
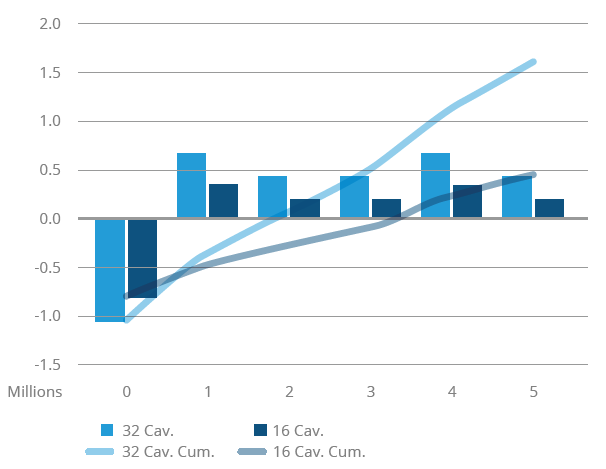
Figure 4: Choosing a 16- or 32-cavity mould.
After the drug has successfully entered the market, production needs to be scaled up in Phase 4. Production capacity can be expanded through repeat moulds, but for most products it is more cost-effective to employ moulds with 48, 64, 96 or 128 cavities. These high cavity moulds significantly improve output and reduce the total cost of ownership. The challenge, however, is to deliver the same product quality in these high-cavity moulds. With more products produced per shot, it is difficult to achieve the same process capability index (Cpk) value. To maintain the same high quality, extreme precision is required in every aspect of the mould, including cavity-to-cavity variation, gate design, gate accuracy and even the cooling channels.
IGS GeboJagema’s approach to tackling this challenge starts with an industry 4.0 production environment. Eliminating human hands from the mould production process allows the company to achieve an extremely low cavity-to-cavity variation. IGS GeboJagema also uses gates that are identical down to a single submicron. This ensures that the resistance is the same across the entire tool during the moulding process and improves the filling balance of the tool.
Additionally, during mould validation, the company uses an advanced version of PRO-OP – software initially developed by Cambridge University (UK) and optimised by IGS GeboJagema for high-precision mould qualification. The program is used to identify the injection-moulding process settings that produce the highest possible quality. In short, through extreme precision mould making, the most cost-effective production can be combined with high quality.
“IGS GeboJagema works together with rapid prototyping and automation equipment partners, allowing OEMs to work with a single partner from Phase 1 to 4.”
AVOID BREAKING UP THE LEARNING CURVE
There is one more best practice for OEMs that want to optimise their product for industrialisation. It is common for OEMs to collaborate with different partners during development. For example, during Phases 1 and 2 they may work with a shop for rapid prototyping soft tools, while working with a larger, industrialisation focused mould manufacturer in the later phases. The downside of this approach is that it breaks up the learning curve. Different teams work on the development of the mould, which inevitably means that learnings and insights are lost when they are passed from one organisation to another. To avoid this, IGS GeboJagema works together with rapid prototyping and automation equipment partners, allowing OEMs to work with a single partner from Phase 1 to 4. As the same team can now collect all the learning and experience, this enables a more streamlined engineering process, resulting in a superior product that reaches the market more rapidly.
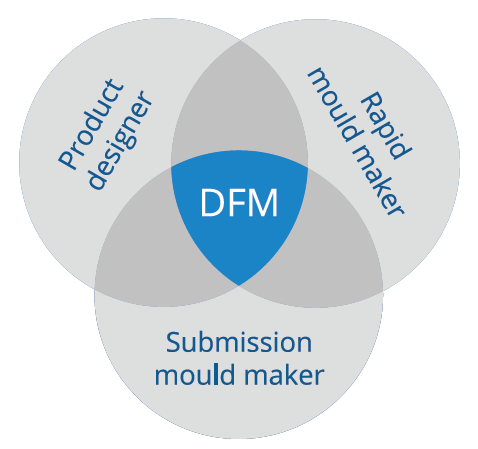
Figure 5: Working together throughout the entire development cycle.
A FORWARD-THINKING APPROACH
In this article, IGS GeboJagema has argued for a forward-thinking approach. By collaborating with an experienced partner throughout the entire development cycle, OEMs can ensure that critical learnings and insights are retained and applied effectively. This approach maximises the potential for cost-effective production and reduces the total cost of ownership and lead times. It simplifies the development process while maintaining the highest standards of quality and performance for the end-user (Figure 5).

