To Issue 151
Citation: Wolf M, Stockton M, “Sustainable Design of a Drug Delivery Device for Biopharmaceuticals”. ONdrugDelivery, Issue 151 (Sep 2023), pp 28–32.
Maike Wolf and Marie Stockton discuss how sustainability is a fundamental strategic priority for Gerresheimer. They also highlight how eco-design principles have been applied to the development of an on-body injector for subcutaneous delivery of biopharmaceuticals.
Medical devices play a vital role in supporting quality of life for patients by delivering essential therapeutic drugs that manage life-threatening diseases. Ensuring that sustainability is integrated into a device’s design and lifecycle is also crucial for enhancing quality of life across broader communities – and the planet.
“The need for sustainable practices in the industry has become vital.”
A HOLISTIC STRATEGY FOR A SUSTAINABLE FUTURE
The manufacturing, distribution, use and disposal of medical devices have a significant impact on the environment.
The need for sustainable practices in the industry has become vital. This is why Gerresheimer has embedded sustainability into its strategic agenda – committing itself to measurable targets.
The company has as its mission to “innovate and deliver for a better life every day”. This mission translates into development of innovative service and product solutions for customers, as well as a focus on how the organisation contributes to improved quality of life for patients, employees, communities and the environment.
To address these topics in relation to sustainability, Gerresheimer has introduced a systematic, integrated approach. The strategy is split into three pillars: GxPure, which focuses on climate and water; GxCare, which encompasses employee satisfaction, health and safety and community engagement; and GxCircular, which sets targets regarding sustainable product design, responsible supply chain management and waste management (Figure 1).
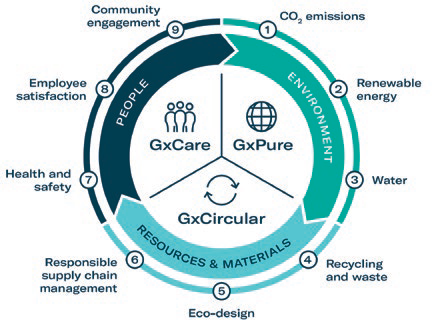
Figure 1: Gerresheimer’s sustainability strategy.
“Designing for sustainability is a key element of Gerresheimer’s GxCircular strategy.”
The company is working continuously towards integrating sustainability into process design, product development and decision making. Gerresheimer has defined a set of measurable targets to ensure that the implementation status of the strategy is transparent to internal and external stakeholders. For example, as part of the GxPure pillar, the goal is to reduce Scope 1 and Scope 2 carbon dioxide equivalent (CO2e) emissions by 50% by 2030 (compared with 2019). One main lever to achieve this is to progressively switch to 100% renewable electricity. The company is already on its way to achieving this target, with 35% renewable electricity currently used across its sites.
With these goals and commitments, Gerresheimer contributes to The 2030 Agenda for Sustainable Development adopted by all United Nations (UN) member states in 2015 and the associated Sustainable Development Goals (SDGs). Gerresheimer is also a member of the Alliance for Water Stewardship (AWS) and, in 2021, the company became a member of the UN Global Compact, committing to its 10 principles on human rights, labour, environment and anti-corruption.
In 2022, Gerresheimer was recognised for its ongoing sustainability efforts with a number of awards and successful ratings from independent bodies. Among these was a gold medal from EcoVadis, a leading independent provider of sustainability ratings. This translates to a top 2% score within the industry. Gerresheimer also achieved an A rating from the Carbon Disclosure Project (CDP) and an MSCI environmental, social and governance (ESG) rating of AA in 2022. Even more recently, Gerresheimer signed a commitment to develop a science-based target according to the criteria defined by the Science Based Targets initiative (SBTi). This means that the company will build on its existing set of climate goals by developing a scope 3 target within the next 24 months.1
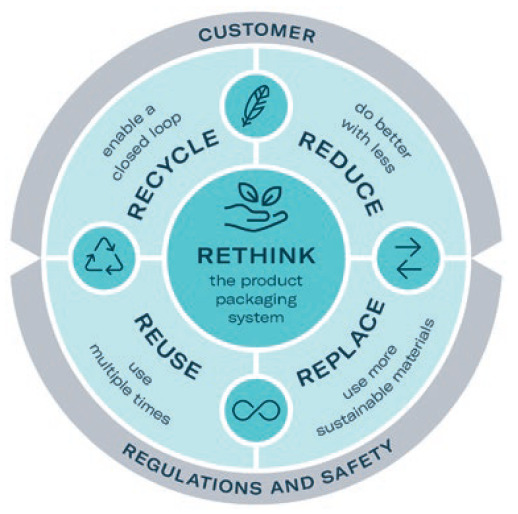
Figure 2: Gerresheimer’s eco-design principles.
ECO-DESIGN PRINCIPLES IN PRODUCT DEVELOPMENT
Designing for sustainability is a key element of Gerresheimer’s GxCircular strategy. The company has committed to incorporating eco-design principles into all new product development projects from 2023. These activities are linked to the commonly used principles of reduce, reuse, recycle and rethink (Figure 2).
One of the products developed by Gerresheimer following these principles is an on-body injector called Gx SensAir® for subcutaneous delivery of biopharmaceuticals in volumes of up to 10 mL. Right from the project concept stage, two key aims were to optimise the patient experience to support quality of life and to decrease the negative impact on the environment resulting from administration of patient therapy.
Designed as a platform device, the Gx SensAir® responds to the growing pipeline of biopharmaceutical drugs for immunotherapy and to treat conditions such as metabolic disease, which may need to be administered at intervals across several months. It has been designed for at-home use, which eliminates the need for a patient to travel to a healthcare provider for drug administration, thus relieving the burden on the healthcare industry and providing more independence to the patient. With less patient travel, the carbon footprint is also decreased.
Following eco-design principles, the developers sought to increase the lifespan of the device and decrease waste by making it partially reusable, while maintaining critical hygiene standards. Finally, consideration was given to the impact of the required cold storage of biopharmaceuticals and how to minimise the related impact along the value chain.
“Subcutaneous self-administration has been shown to improve patient quality of life and reduce treatment time.”
REDUCTION OF CO2 FOOTPRINT WITH SELF-ADMINISTRATION
Patient-oriented, cutting-edge medicine in hospitals is fundamentally accompanied by high energy consumption. In Germany, hospitals are among the sixth-largest energy consumers in the trade, services and commerce sector. As an illustrative example, if the total energy consumption of a hospital is divided by the number of beds, the energy consumption represented by one hospital bed is, on average, equivalent to that of four family homes.2
To date, many patients with a wide variety of indications have to endure long and stressful intravenous infusions in a hospital setting to treat their condition. Homecare enables patients to return home as quickly as possible – for example, after a hospital stay – even if they still require certain therapy and care measures due to their illness. Other therapies may not need to be administered in a hospital or other care facility at all, but could be self-administered subcutaneously at home after a single visit to the doctor.
Subcutaneous self-administration has been shown to improve patient quality of life and reduce treatment time. To evaluate patient preference, Gerresheimer conducted a small-scale patient preference study of 40 breast cancer patients in Europe and the US. The results showed that 68% preferred the idea of using an on-body injector versus standard subcutaneous injection and over 70% were in favour of self-administration at home (Figure 3).3
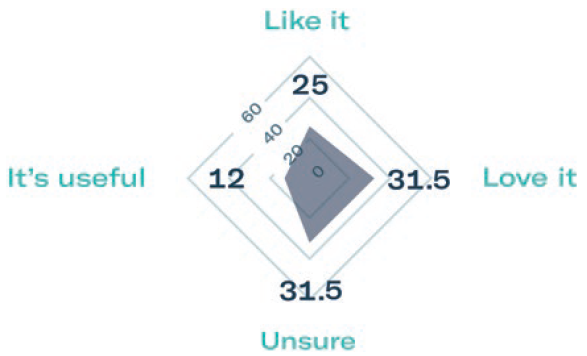
Figure 3: Results of patient preference study.
In terms of impact on CO2 footprint, using the example of a 28-year-old Swiss patient suffering from familial hypercholesterolemia since childhood, calculations can be made to determine an approximate decrease in carbon footprint due to at-home therapy administration. With hypercholesterolemia, there is too much cholesterol in the blood, which can cause deposits in the vessels and lead to atherosclerosis-related cardiovascular disease.
By 2025, refractory hypercholesterolemia will be the predominant indication in approximately 4.3 million people.4 Treatment with the fully humanised monoclonal antibody evinacumab has been shown to inhibit lipoprotein lipase and endothelial lipase and would be applied subcutaneously once a month for a period of approximately 17 months.5,6
The average person living in Switzerland generates 14 tons of CO2 per year, according to the my climate web page. By not travelling to hospital by car for treatment once per month for the 17-month treatment period, the patient would save 0.208 tons of CO2. If all 4.3 million potential patients worldwide diagnosed with hypercholesterolemia used a self-administration device such as Gx SensAir®, there would be potential savings of an average annual footprint of 30,735 people.
“By embracing sustainability, the medical device industry can mitigate resource depletion, reduce waste generation, maximise energy efficiency and minimise CO2 emissions.”
REUSABLE/DISPOSABLE CONCEPT
One part of the Gx SensAir® medical device – the reusable module – contains all the electronics and the air pump. The other part – the disposable module – is disposed of after each course of therapy. The disposable module includes the fluidic path and needle, which injects the drug automatically. A third element of the design is the primary packaging, in this case, a cartridge that is prefilled with a biologic drug. The cartridge is of standard size with different fill volumes, which avoids additional customisation. The three elements are clicked together by the patient before applying to the body (Figure 4). As the reusable module does not contain a needle, it does not require sterilisation – which reduces energy and water consumption.

Figure 4: Gx SensAir® reusable concept.
The sterilised disposable module, including needle and cartridge, is designed for disposal according to country-specific regulations for waste management of “sharps”. As the device has been designed with minimal materials and without glue or screws, the reusable electronics module can be easily recycled once it has reached the end of its life.
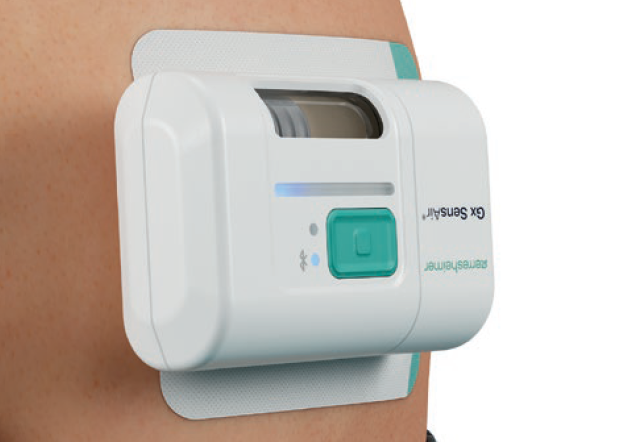
Figure 5: Gx SensAir® automatic subcutaneous drug administration.
PATIENT ADVANTAGES OF REUSABLE CONCEPT
In addition to the sustainability aspects of the Gx SensAir®, additional benefits of having a reusable module include those associated with the integration of additional electronics that would not make financial or environmental sense in a single-use device. This includes indicators to show injection progress, occlusion detection, audible signals and automatic needle insertion (Figure 5). All of these features enhance usability and enable at-home administration to limit otherwise unnecessary visits to a healthcare provider. Timing and duration of drug delivery according to therapy needs and drug features, such as viscosity, can also be stipulated for an optimal balance of patient comfort and effective delivery. Additionally, the Gx SensAir® reusable module has Bluetooth connectivity, which can deliver advantages to the patient and valuable data to a healthcare provider, potentially enhancing adherence.
When considering the patient-loading concept that supports sustainability, it was critical to understand the voice of the patient. As the US FDA has said, “Only patients live with their medical conditions and make daily choices regarding their healthcare. Their voices and perspectives are critical to understanding the impact of medical devices.”7 In a usability evaluation for the Gx SensAir®, test subjects who had never previously used an on-body drug delivery device were asked to simulate assembly, drug administration and disassembly. The goal was to observe users and discover any unanticipated use errors, optimise the user experience and foresee misuse.
A few errors were observed and the design was optimised accordingly to further simplify the assembly and usage. Overall user feedback was that the product was “easy to use” and that self-administration of therapy “can be done before breakfast, half asleep”. Overall, the study showed that the reusable concept, in addition to contributing towards sustainability objectives, was also simple to use for the patient.3
REDUCED REQUIREMENT FOR COLD-CHAIN LOGISTICS
Biopharmaceuticals, such as monoclonal antibodies, require refrigeration, which impacts the entire value chain. In some injectors, where the primary packaging is already integrated into the delivery device, it is necessary to sterilise, package and cold store the entire device. This increases CO2 emissions throughout the value chain, from manufacturing, to fill-finish, through distribution, to the pharmacy, healthcare provider and, in the case of self-administration devices, the patient’s own home. With the Gx SensAir®, the patient-loading concept means that only the cartridge needs to be refrigerated, which significantly reduces the need for refrigeration space. This corresponds to Gerresheimer’s goals by reducing energy consumption and associated emissions throughout the value chain.
IMPORTANCE OF AN INTEGRATED APPROACH TO SUSTAINABILITY
Gerresheimer’s strategic commitment to sustainability and innovation is only part of the story. To be able to fully implement its sustainability goals, collaboration is key. Support from internal stakeholders, external investors, customers and suppliers is needed. When working with suppliers, Gerresheimer assesses compliance with environmental standards and regulations, as stipulated in its supplier code of conduct. All strategic suppliers will be required to acknowledge the code of conduct by 2024.
Gerresheimer also works closely with customers not only to comply with legal requirements and patient usability but also to proactively and systematically integrate eco-design principles into product design throughout the value chain. With this approach firmly in mind, Gerresheimer aims to combine skills and abilities and create synergies to achieve innovative and integrated solutions that meet customer and patient needs. Providing data and ensuring transparency along the value chain also supports the sustainability efforts of customers.
By embracing sustainability, the medical device industry can mitigate resource depletion, reduce waste generation, maximise energy efficiency and minimise CO2 emissions. Sustainable practices and patient-centric design can be complementary goals, ensuring environmentally responsible solutions that also enhance the patient therapy experience.
Find out more about Gerresheimer’s on-body SC delivery systems at: www.gerresheimer.com/en/drug-delivery-systems/platform-solutions/infusion-systems.
REFERENCES
- Annual Report, Gerresheimer, February 23, 2023.
- Werner JA (Ed), Struchholz A (Ed), Geuting A (Ed), “Smart Hospital: Digitale und empathische Zukunftsmedizin”, Part III, Chapter 7 (“Smart Hospital und Nachhaltigkeit”), p 163, MWV Medizinisch Wissenschaftliche Verlagsgesellschaft, 2020. (ISBN: 978-3-95466-495-5)
- Abedian R, “Patient-Centered Approach to Development of Novel On-Body Drug-Delivery Solutions for Injectable Biologics: A Study with Multiple Target Groups”. PDA Universe of Pre-Filled Syringes & Injection Devices Conference, Palm Springs, CA, US, October 18-19, 2022.
- “Homoczygous familiar hypercholesteria”. GlobalData.
- Highlights of Prescribing Information, Package Insert, EVKEEZA™ (evinacumab-dgnb) injection for intravenous use, Regeneron, March 2023.
- Jeraj N et al, “Treatment of Homozygous Familial Hypercholesterolemia with Evinacumab”. CJC Open, 2022, Vol 4 (3), pp 347-349.
- “Patient Preference Information (PPI) in Medical Device Decision Making”. Web Page, US FDA, July 3, 2023.

