To Issue 164
Citation: Gross C, Tunkel M, “Symbioze®: Large-Volume On-Body Injector Platform Fostering Patients’ Experience” . ONdrugDelivery, Issue 164 (Sep 2024), pp 48–51.
Cécile Gross and Mark Tunkel discuss the how Nemera‘s on-body injector platform – Symbioze® – has been developed by drawing on a combination of lessons learned from the history of injection devices and the company’s extensive experience developing drug delivery devices for the parenteral route.
When considering the delivery of injectables since the first human insulin injection more than 100 years ago, it is clear that the journey from then until now has been paved with many discoveries and improvements – especially when it comes to lessening the burden of disease on patients. From a drug perspective, many developments have led to an increase in the number of pathologies treated via this route, including rare diseases, and an expansion in the therapeutic areas addressed by to the rise of biologics. From a delivery device perspective as well, evolutions have been steady, much to the benefit of patients.
FROM GLASS SYRINGES TO PATCH PUMPS
Returning to the insulin example, bulky reusable glass syringes were replaced by thin disposable plastic syringes with the primary benefit being a reduction in the needle thickness, which translated into less pain for patients during their multiple daily injections.1 Even if, from a cost perspective, a prefilled syringe or the combination of syringe and vial were – and still are – attractive, administering an accurate dose, carrying the device and, above all, dealing with the psychological aspects of coping with disease remained problematic.
The arrival of pen injectors in the 1980s enabled patients to overcome these factors. Dose accuracy was improved, followed by clinical outcomes and, ultimately, patients’ adherence and acceptance. With regard to reducing the pain, pen injectors only require a low injection force to administer the dose thanks to their very thin single-use needles. Needle phobia is also more easily avoided with this type of needle compared with standard syringe needles.
Another positive aspect is the flexibility offered to patients – there is a variety of choices between reusable and disposable devices, between colours and shapes for younger patients, and between connected devices or standalone ones. When connectivity is desired, the technical solution selected to enable it becomes part of the patient’s continuous glucose management routine, often increasing compliance overall. Finally, the pen shape is discrete and easy to carry, helping with the aforementioned psychological aspects of the disease.
The next jump occurred in the 1990s with insulin pumps for continuous delivery, which have become small, compact, handy and effective. Connectivity offers the possibility to “close the loop” for blood glucose monitoring. Needless to say, managing such a pump requires training, involvement and financial capability on the part of the patient, but it definitely offers lifestyle flexibility without compromising reliability in dose delivery.
Recently, patch pumps seem to have begun replacing infusion sets. These devices are attached to the patient’s skin via an adhesive, which is less invasive than tubing or a catheter. Beyond the commonly expected connectivity features, these pumps are usually equipped with advanced algorithms that enable patients to monitor their condition more closely.
In the same way that solutions provided to patients with Type 1 diabetes later spread to those with Type 2, the evolution in the treatment of diabetes and its comorbidities has led the way for other drugs and therapies. The current developments in injectables are fully benefiting from this evolution. The latest example is on-body delivery systems, which aim to provide bolus delivery of biologics in the fields of immunology, oncology and haematology.
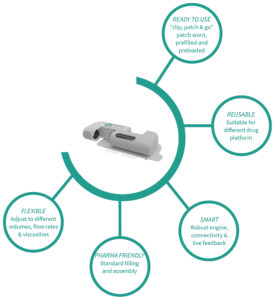
Figure 1: Symbioze® on-body injector key features and benefits.
FROM PATCH PUMPS TO ON-BODY INJECTORS: SYMBIOZE®, AN IDEAL SOLUTION FOR PATIENTS
In order to offer a comprehensive device range for injectables, Nemera has developed Symbioze® (Figure 1) – an on-body injector platform addressing both the challenges of large-volume delivery and sustainability with its reusable-disposable design. Building on the history of injectable pumps discussed here, along with the company’s extensive experience with pen injectors, patient-friendly features are an integral part of the Symbioze® platform.

Figure 2: Human factors studies were conducted by Insight by Nemera.
Starting with the patch itself, the supplier has been carefully selected for its track record and ability to customise its shape to the device. The shape has been developed with a focus on applying insights gained from the in-house human factors studies conducted by Insight by Nemera (Figure 2). Work is currently in progress to optimise the usability of Symbioze® by limiting the number of user steps and providing comprehensive instructions for use (IFU), which is expected to increase patient adherence (Figure 3).

Figure 3: Clear IFU are crucial for therapy success.
“The drug is delivered via a soft cannula, which is reminiscent of the tubing used in older devices and increases patient comfort.”
As is standard for patch pumps, Symbioze® has automatic insertion and retraction of the needle, which makes the safety cap a must to avoid needlestick injuries. However, the drug is delivered via a soft cannula, which is reminiscent of the tubing used in older devices and increases patient comfort. There is also an important feature that increases patient safety, whereby the drug is not delivered if the device is not worn properly – this is expected to influence the clinical outcomes to follow a positive curve. This may also avoid high-value drug loss for pharma companies.
Being able to offer a seamless and fluid injection experience encouraged the design team to limit the number of buttons on the interface as well as to manage connectivity and software management remotely from an app or the cloud for the purpose of enhancing the patient’s engagement. To foster patient adherence, the device must not be too complicated to handle, and the guiding instructions must speak by themselves.
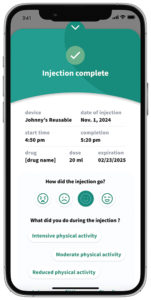
Figure 4: Symbioze® offers the possibility for connected accessory support.
One of Nemera’s offerings is connected device UI/UX design. The company has a comprehensive understanding of user needs that informs the development process to determine how to balance development of the device, sensor systems, software and digital platforms to best support the user. Nemera applies this to the development of the device, the mobile application and software development, ensuring that requirements are driven by an understanding of user needs – balancing those with the capabilities of the technology. This design and engineering capability is used to deliver smart solutions that make a real difference to patients’ lives, including in combination with Symbioze (Figure 4).
“Nemera’s solution was to add a membrane at the crimping place to control the fluid path opening.”
Although sterility and contamination are not an issue in other injection devices, they become a consideration for Symbioze because of its reusability. How can the sterility barrier be ensured when assembling two separate parts? The design team had to overcome this challenge to avoid the manipulation of the primary drug container – a large-volume glass cartridge – by patients. The choice not to have the naked cartridge separated from its housing has been driven by Nemera’s wealth of experience in safety systems for prefilled syringes. Many of its pharma partners are offering high-value drugs in this format, so it is critical for self-injecting patients not to have to worry about correctly positioning the drug container.
Preserving the drug and its container has been a priority during the early phases of the platform development. Nemera’s solution was to add a membrane at the crimping place to control the fluid path opening. This solution has been validated through a series of tests (Figure 5), some of which were conducted with the help of the Hôpitaux Civils de Lyon (France), Lyon’s biggest public hospital network.
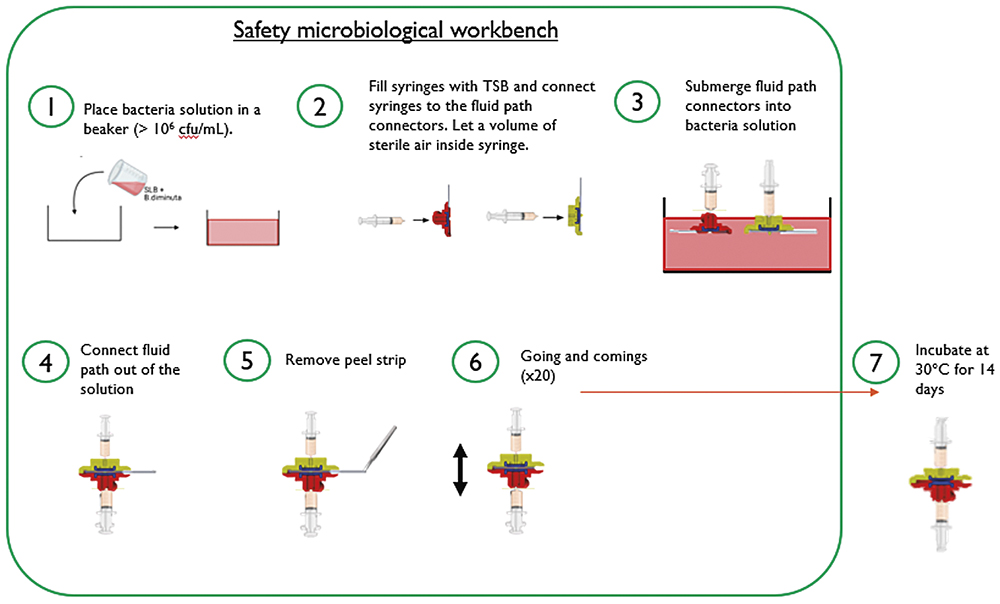
Figure 5: Testing methodology for ensuring the sterility of Symbioze®.
Overall, Symbioze® is a pure combination of the lessons learned from the history of injectable delivery devices and Nemera’s experience developing combination products. It is also an ideal representative of Nemera’s motto: “We put patients first”.
“By building on the platform’s solid foundation, Nemera can ensure that Symbioze® platform is tailored to the specific needs of the target patient population, drug characteristics, delivery time and regulatory requirements.”
FROM PLATFORM TO MARKETED COMBINATION PRODUCT – THE VALUE OF PARTNERING WITH AN INTEGRATED SERVICE PROVIDER
Partnering with Nemera can simplify the process of integrating Symbioze® with a drug product, accelerating the development of combination products and expediting time to market while managing the complexities associated with developing on-body injectors. By building on the platform’s solid foundation, Nemera can ensure that Symbioze® platform is tailored to the specific needs of the target patient population, drug characteristics, delivery time and regulatory requirements. The Insight byNemera development and consulting organisation provides comprehensive services and capabilities covering critical areas essential to the success of combination products:
- Functionality testing, performance testing, analytical services and design verification
- Human factors management and design validation
- IFU and packaging development
- Regulatory strategy and submission
- Drug/device assembly and packaging support.
Collaborating with an integrated partner offers several advantages, including a patient-centric approach that considers the customisation of the device and its supporting elements, such as IFU, packaging, digital experiences and training solutions to increase adherence and engagement.
Nemera recognises that navigating the complex ecosystem of combination products to achieve market success can be challenging. As such, its services are designed to align with the expectations of various stakeholders, including healthcare professionals, networks, payers and regulators. To address the challenges of drug delivery device development, Nemera applies its end-to-end expertise throughout the process, ensuring consistent execution and minimising the risk of essential information being lost at different stages or when working with multiple partners. With its experience in managing complex user needs and regulatory requirements, Nemera can accelerate a project’s time to filings and market entry.
Ultimately, the value of Nemera’s integrated services lies in the flexibility they offer, allowing customers to focus on their core business of drug discovery and development. As a partner and extension of customer teams, Nemera delivers best-in- class solutions, ensuring no compromises are made during collaborations.
Symbioze® is a trademark of Nemera La Verpillière SAS.
REFERENCE
- Kesavadev J et al, “Evolution of Insulin Delivery Devices: From Syringes, Pens, and Pumps to DIY Artificial Pancreas”. Diabetes Ther, 2020, Vol 6(11), pp 1251–1269.

