Citation: Kriheli M, “The Need for CSTDS in both Manual and Automated Compounding”. ONdrugDelivery Magazine, Issue 105 (Feb 2020), pp 68-71.
Marino Kriheli discusses the benefits of using closed system transfer devices (CSTDs) in both manual and automated hazardous drugs compounding.
Healthcare workers may be exposed to hazardous drugs (HDs), often chemotherapy medications, at many points during their manufacture, distribution, storage, transport, compounding and administration, as well as during waste handling and care of treated patients.1 The risk of exposure is, therefore, not limited to those directly involved with the drugs, such as pharmacists or nurses. It extends to all workers, including those involved in the maintenance and repair of equipment in the compounding and administration environment.
This has been confirmed in a clinical study which found drug contamination on select surfaces at every stage of the medication system, which it said indicated the existence of an exposure potential throughout the facility – potentially leaving “up to 11 job categories per site [that] may be at risk of exposure at some point during the hospital medication system”.2
“The consequences for not properly protecting staff members can be severe.”
According to the US National Institute for Occupational Safety and Health (NIOSH), approximately eight million US healthcare workers are at risk of exposure to HDs, while the European Commission estimates that some 20 million European healthcare workers are at similar risk.
The consequences for not properly protecting these staff members can be severe. NIOSH has demonstrated that exposure to HDs can produce negative health outcomes, including abdominal pain, nausea and vomiting, diarrhoea, hair loss, dermatitis, irritation of skin and eyes, irritation of mucous membranes and menstrual cycle disruption. In more extreme cases, exposure can result in birth defects, miscarriages and even some forms of cancer.
As a result, health agencies worldwide have created safety guidelines for interacting with HDs. NIOSH recommends that, in tandem with other safety measures, healthcare workers use a CSTD, throughout the HD-handling process. And the US Pharmacopeia (USP) recommends that CSTDs be used as supplemental engineering controls in USP 800, which came into effect in December 2019.3 The UK’s Health & Safety Executive (HSE) recommends the use of fully closed systems where reasonable4 and EU-OSHA in the EU lists closed systems in its “hierarchy of prevention”.5
The importance of CSTD use was further demonstrated in a study by Vyas in 2013 which showed that CSTDs can reduce HD contamination – and CSTDs are increasingly becoming the standard of care. However, the need for better protective measures will increase over time as the number of patients requiring these drug therapies continues to grow.
ONLY FULLY CLOSED SYSTEMS ARE EFFECTIVE
CSTD use is becoming standard practice across healthcare markets as global bodies follow US examples, including NIOSH and USP in championing the use of CSTDs, especially given the improvements in the available products over time. The original design of CSTDs took the correct approach by mechanically preventing escape of hazards out of the system as well as stopping environmental contaminants entering the device. These original “barrier systems” isolated people from hazards while also protecting the sterility of HDs.
“If an automated compounding system does not use CSTDs throughout the process, the same dangers of environmental contamination in manual compounding still exist.”
Subsequently, alternative filter-based systems have emerged. However, this design approach is inherently flawed due to the reliance on filtration to separate hazardous materials from non-hazardous. For example, even the largest drug vapour is at least 1,000 times smaller than the filter paper used in many filter-based systems. Such systems also tend to rely on the inclusion of a charcoal filter. However, use of charcoal filters includes a number of variables and, while they may be effective at absorbing certain substances, they may not be effective in preventing other materials passing through. An additional concern with charcoal filters is that, over time, they become saturated – which will further impact their effectiveness. Furthermore, while NIOSH concedes that filter systems can be effective for certain drugs that have no potential to generate vapour, there are currently more than 200 drugs identified on its list of HDs, the majority of which do release vapours.
The Japanese NIOSH and the International Society of Oncology Pharmacy Practitioners (ISOPP) have similarly stated that filter systems can become saturated and have varying efficiencies.6 Both bodies have concluded that filter-based CSTDs do not meet the definition for what should be considered a closed system.
In contrast, barrier systems have several distinguishing advantages which set them apart from filter-based devices, with the most obvious being that closed systems are completely sealed and do not allow dangerous vapours to escape. Being completely closed also includes needle safety, as the needle is not exposed at any point of connection and disconnection – preventing the risk of needlestick injuries.

Figure 1: The Equashield CSTD,
with encapsulated syringe barrel
and a metal plunger rod.
EQUASHIELD’S KEY INNOVATIONS
When Equashield entered the CSTD market over a decade ago, the company’s goal was to streamline and improve the original CSTD design concept, offering superior safety and ease of use for the compounding and administration of HDs.
The Equashield CSTD was developed to cover more routes of exposure than alternative systems. It features an airtight encapsulated syringe barrel and a metal syringe plunger rod which runs through the centre of the syringe chamber to prevent contamination from drug residue on the syringe barrel wall. The plunger rod is also wiped clean before being withdrawn from the barrel at the encapsulation point (Figure 1).
Equashield’s key innovation compared with the original CSTD design can be seen in its pressure equalisation mechanism. Rather than using an external balloon to store sterile or contaminated air, Equashield designed the syringe barrel itself to store sterile air before use and to contain hazardous materials within the chamber after the HD has been injected. This air-to-liquid exchange and containment is possible through the two needles placed within the syringe.
To prevent leaks at connection points, Equashield uses a double membrane locking system to prevent liquid droplets escaping between connections. The rubber membranes are located at the end of the CSTD and on the vial adaptor, locking together first and then allowing the needle to pass through the double layer to reach the drug in the vial. The needle then retracts through the double membrane upon removal and is never exposed throughout the process (Figure 2).
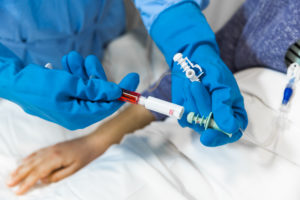
Figure 2: Equashield’s “needle-free” connections.
Lastly, just a single motion is required to connect and disconnect any Equashield components, which enables quick transfers with minimal force for significantly improved ease of use by pharmacists and nurses who handle the compounding and administration of HDs.
CRUCIAL FOR SAFE AND EFFICIENT AUTOMATION
As in many other industries, automation has entered the drug compounding space, bringing added benefits such as increased efficiency and patient-specific dose preparation. However, while it has been established that CSTDs are an integral component of manual HD compounding, integration of CSTDs is often overlooked in robotic compounding, as the assumption is that the robot contains any escaped hazards within.
“While traditional compounding robots are a step in the right direction, they are not able to prevent contamination.”
However, just as there would be issues of HD exposure without using a CSTD working in a biological safety cabinet or within an isolator, if an automated compounding system does not use CSTDs throughout the process, the same dangers of environmental contamination in manual compounding still exist.
While traditional compounding robots are a step in the right direction, they are not able to prevent contamination. Residue has been found on intravenous therapy bags, syringes and working surfaces for a number of reasons, such as the syringes being open at certain points in the compounding process. Prior to attaching the needle to the syringe, or before a syringe with a full dose is capped, HDs are fully exposed and able to escape into the environment. Therefore, the prepared doses that are produced by these systems may be saturated in HD residue, presenting considerable risk to healthcare workers in the vicinity.
With this in mind, both manual and automated compounding need to be treated equally when it comes to HD exposure, and CSTDs must be incorporated for both manual and automated processes to minimise environmental contamination.
NEW GENERATION OF SAFE AND EFFICIENT AUTOMATED HD COMPOUNDING
This is why we think the Equashield PRO – the first ever CSTD-enabled fully automated compounding system – is the safest and most efficient HD compounding robot on the market. Unlike earlier systems, which not only fail to prevent contamination but also tend to be slow and inefficient, the PRO was designed to address these shortcomings and re-imagine the automation process safely and efficiently (Figure 3).

Figure 3: The Equashield PRO fully automated compounding system.
A major shortcoming in the design of the original compounding robots is the reliance on one robotic arm alone to perform the majority of varied tasks. Equashield PRO was designed to operate more like a factory line, using eight simultaneous workstations to complete the compounding process. Each station is an effective “expert” in its role and performs its task quickly and efficiently, shortening the length of the entire process.
Another significant issue with traditional compounding robots is the “grabbing” mechanism. Because each drug vial is a different size, a robotic arm must spend extensive time identifying the right point to pick up the vial to safely withdraw a dose. This time-consuming process is compounded by the lengthy processes of needle insertion into the vial. When a traditional robot grabs a syringe or drug vial, it does not always pick up the piece in the same spot. As such, it takes more time for the robot to then determine where the needle is, where it needs to be inserted and how deeply to insert it to withdraw the right amount of the drug, without wasting any of the liquid.

Figure 4: The Equashield PRO vial-grabbing mechanism and verification system.
Because the PRO uses CSTDs, the syringes and vials all use uniform vial adaptors. This allows the PRO to move much faster. It is able to pick up any size of vial in the same manner, thanks to the vial adaptor.
The PRO is also able to verify the drug and dosage with visual verification software and efficiently bring it to the next station (Figure 4). The PRO can be used for high-throughput, patient-specific dose preparation, as well as batch compounding. Equipped for both tasks, it can store more than 50 syringes and 70 drug vials, allowing it to produce over 60 individual doses per hour. The PRO also offers medication error control by using verification software for each dose and can detect any bubbles in the syringe, which could result in inaccurate doses. The PRO’s factory-style line-up is all housed in a machine comparable in size to standard biological safety cabinets.
CONCLUSION
It is vitally important that healthcare facilities use clinical judgement when deciding on which closed system to implement in order to properly protect healthcare workers. After many years of research and education, we are at the stage when CSTDs are becoming a best practice in HD handling, supported by regulation. The future of compounding, however, is still developing as automation technologies begin to take the stage. As automation becomes more prevalent, we must ensure that the protective principles that are currently in place for manual compounding are retained with automation – with CSTDs as a central part of the compounding process.
REFERENCES
- “Preventing occupational exposure to antineoplastic and other hazardous drugs in health care settings”. US NIOSH alert, 2016.
- Hon C et al, “Occupational Exposure to Antineoplastic Drugs: Identification of Job Categories Potentially Exposed throughout the Hospital Medication System”. Saf Health Work, 2011, Vol 2(3), pp 273–281.
- “Hazardous Drugs – Handling in Healthcare Settings”. USP General Chapter, 2017.
- “Safe Handling of Cytotoxic Drugs in the Workplace”. UK HSE.
- “Dangerous Substances”. EU-OSHA.
- “Considerable Controversy Accompanies NIOSH Move Toward a Universal Protocol for Testing CSTDs”. Pharmacy Times, May 2017.


