To Issue 162
Citation: Leipold F, Vrijens B. “Smart MEMS Cap for Sharps Waste: A Simple and Elegant Solution to Clinical Trial Adherence Monitoring”. ONdrugDelivery, Issue 162 (Jun 2024), pp 22–26.
Frank Leipold and Bernard Vrijens discuss Haselmeier & AARDEX’ s collaboration to develop the MEMS Smart Cap – a smart solution for data collection during clinical trials to enable an accurate assessment of participant nonadherence.
“Trial participants failing to take their medications as specified can lead to biased results, which can lead to significant delays and additional costs.”
Nonadherence is one of the most critical challenges facing clinical trials – as many as 50% of patients are reported not to adhere to the treatment regimens laid out in trial protocols.1 Trial participants failing to take their medications as specified can lead to biased results, which can lead to significant delays and additional costs. This challenge is compounded by the difficulties in recruiting participants, especially for rare and orphan diseases, and the ever-present pressure to reduce time to market. To meet this challenge, Haselmeier, a medmix Brand, and AARDEX Group have joined forces to optimise dose exposure and speed up clinical trials.
THE CHALLENGE OF NONADHERENCE
How can nonadherence be tackled? Often, it simply isn’t, with many trials relying on self-reporting, that fails to detect it. It is clear that a better approach is required, as nonadherence reduces a study’s ability to determine the new drug’s dose-response relationship. In turn, this leads to longer trials that require larger patient sample sizes and place unnecessary burdens on healthcare systems. In the worst-case scenario, a pharma company could decide to give up on a life-saving drug, based on weak but flawed data.
Rare and Orphan Drugs
These unmet challenges bite particularly hard when coupled with another trend in drug development: drugs being developed to treat increasingly rare indications. According to data from Trialtrove, clinical trials for drugs targeting rare or orphan diseases have gone from 1,236 in 2010 to 2,335 in 2022 – an 89% increase (Figure 1). Currently, there is a great deal of interest across the industry in using biologic medications to treat new disease areas, with many small biotech companies starting up with a tight focus on a specific disease or area, such as the central nervous system. However, these drugs are often difficult and expensive to manufacture, as well as challenging to deliver.
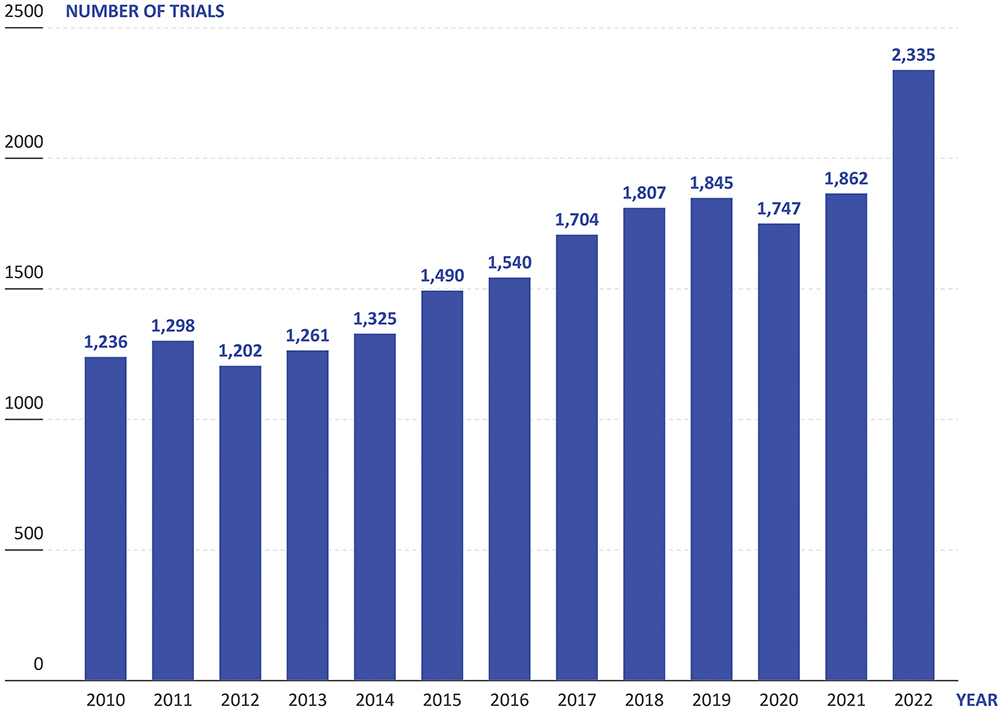
Figure 1: In recent years, the proportion of new drug trials that are for rare and orphan diseases (Source: Trialtrove).
The development of drugs for rare diseases is challenging and requires different development and regulatory approaches. These drugs qualify often for accelerated approval based on limited evidence and small sample size. Confirmatory evidence is then required from post-approval pragmatic trials in which reliable information on drug exposure is required for robust assessment of efficacy and safety in real-world settings.
Biologics are most frequently administered via the parenteral route, being injected either intramuscularly or subcutaneously to avoid first-pass metabolism in the gastrointestinal tract damaging the fragile proteins. However, these injections often require long hold times or larger needles to handle the volumes and viscosities of biologic formulations, which makes these injections unpleasant for patients. This can increase the chance of patients becoming nonadherent – especially in a clinical trial where they are uncertain as to the therapeutic benefit and safety of the medication.
“Decentralised clinical trials have become a necessity for reaching minimum recruitment targets, but this too makes it harder to monitor and enforce adherence.”
It then becomes clear how these two trends interact. With biologics targeting rare diseases with increasingly small patient populations, the difficulty in recruiting for clinical trials increases in tandem, along with the need to retain them. Decentralised clinical trials have become a necessity for reaching minimum recruitment targets, but this too makes it harder to monitor and enforce adherence. At the same time, the reduced number of patients makes high levels of adherence even more critical, as each individual patient has a greater effect on the dose-exposure-response relationship data.
Burden on Healthcare Systems
The number of new drug candidates has more than tripled over the last 20 years to over 21,000 in 2023 (Pharma-projects data, 2023), which is increasing the number of ongoing clinical trials (Figure 2). Simultaneously, the healthcare sector is finding it increasingly challenging to provide the capacity to conduct these trials, with many placed under strain from the higher healthcare needs of ageing populations and staff turnover, amongst other factors. On top of this, pragmatic clinical trials are running for longer, with more complex protocols, while sponsors are presenting ever more stringent demands on recruitment numbers and eligibility criteria. Furthermore, incentive models for healthcare professionals (HCPs) have changed, making working on clinical trials less appealing than it has been historically.
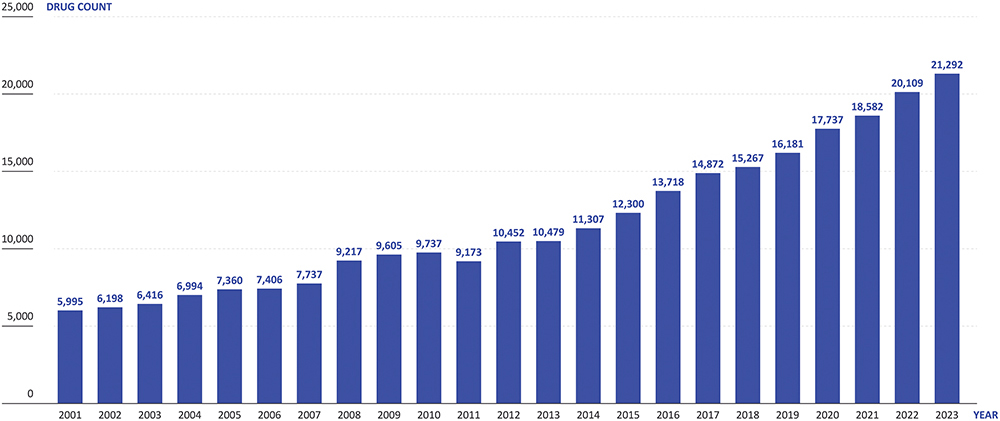
Figure 2: The number of new drug candidates in the pharmaceutical pipeline has more than tripled over the past two decades. (Source: Pharmaprojects)
The Move to Self-Administration
Recognising the burden being placed on healthcare systems, the pharmaceutical industry has been making a concerted push towards developing therapies suitable for patients to self-administer in their own homes, alleviating the need for them to visit clinics and receive the direct attention of an HCP. Whilst this is clearly a positive and necessary move, it does make monitoring and confirming adherence even more challenging – if there is no HCP present during administration, it falls entirely on trial participants to adhere to the correct self-administration procedure and report on it.
One solution to the problem of nonadherence for self-administered therapies that has been gaining traction in the industry is the implementation of connected drug delivery devices. In principle, the idea is sound – connectivity can enable remote monitoring of trial participants, allowing researchers to gather objective data on drug usage. However, this approach is often made overcomplicated, requiring trial participants to engage with a companion app that is linked to their connected drug delivery device, which then links to their smartphone or similar device, issuing reminders along with additional features, whilst automatically logging data that it will then send back to a portal accessible by the researchers. In practice, the extra burden that using these apps presents causes some trial participants to cease using them as the trial progresses, compromising data completeness and losing any benefit to adherence they might have theoretically offered.
With this in mind, is connectivity a dead end in the search for a solution to the nonadherence problem? Not at all – however, it requires a different approach to yield the best results. In order to gain the benefits connectivity can offer to adherence monitoring in self-administered trials, the connectivity features need to be built into the expected use of the medication, without any additional steps. As far as the patient is concerned, there should be minimal difference between the connected and non-connected solution – the monitoring occurring entirely in the background and not intruding on their experience.

Figure 3: AARDEX’s MEMS smart cap.
THE HASELMEIER AND AARDEX SMART MEMS CAP SOLUTION
To solve the challenge of applying connectivity to adherence monitoring, Haselmeier and AARDEX have come together to scale up a proven approach that can integrate seamlessly into the typical patient experience. Combining Haselmeier’s drug delivery engineering expertise with AARDEX’s trusted and established MEMS® system, the two companies have developed a solution for collecting reliable data on medication usage during clinical trials for single-use disposable injection devices.
The Haselmeier and AARDEX solution uses a standard, non-connected single-use injection device, such as a prefilled syringe or disposable autoinjector. The process is kept as simple as possible, with the patient using their injection device as usual. When the patient has completed their injection, they must dispose of the device in a supplied smart sharps waste container; using AARDEX’s MEMS system, connectivity is integrated into a smart cap (Figure 3), logging and transmitting the time and date of disposal as the patient removes and replaces the smart cap, along with tracking the total number of device disposals (Figure 4). This data is then recorded for the trial researchers, who can then respond accordingly to patients’ adherence based on objective data.
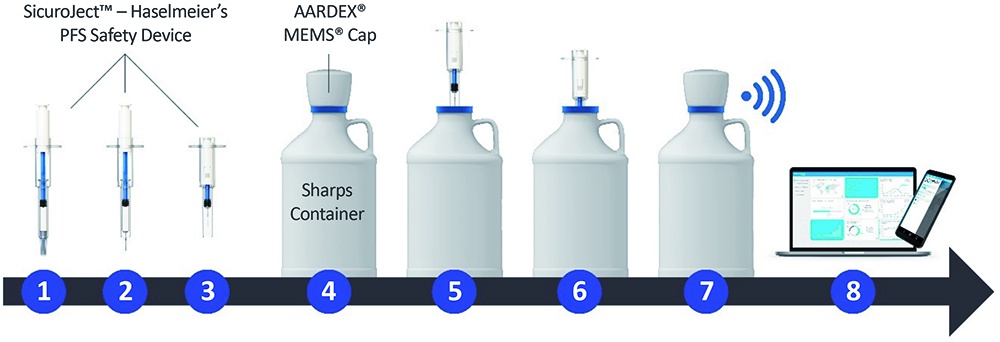
Figure 4: How the Haselmeier and AARDEX smart MEMS cap solution works: (1) Safety device & PFS before injection, (2) Patient removes needle cap, (3) Patient self-injects and needle withdraws into the device, (4) Sharps container with MEMS cap, (5) Patient removes MEMS cap, (6) Patient disposes of the safety device in the sharps container, (7) Patient closes sharps container and the MEMS cap automatically records date and time, (8) MEMS cap transfers data automatically to mobile app and/or MEMS reader.
“Taking an approach where the connectivity is included not in the device, but instead in a smart cap on the sharps container, comes with numerous benefits over a connected-device-based approach.”
Taking an approach where the connectivity is included not in the device, but instead in a smart cap on the sharps container, comes with numerous benefits over a connected-device-based approach. Firstly, as already discussed, this method requires no additional effort from the patient, either in onboarding them on how to use a connected device or in additional steps required during self-administration – they can use a standard disposable device they are already familiar with and the adherence monitoring happens automatically without their input.
Secondly, Haselmeier and AARDEX’s MEMS smart cap saves the significant amount of time and money that would need to be invested into developing and verifying a connected device. Whilst there is certainly potential in connected devices, they add significant complications and challenges to the device design process. For example, electronic components must be included, which require materials and expertise that may not be readily available to a drug delivery device design team, as well as needing to find space in the device for a power source and connectivity infrastructure, all of which takes time and money. This complexity is particularly problematic in the early phases of drug development when speed is essential.
Additionally, adding electronics to a drug delivery device significantly increases its carbon footprint, which, combined with the cost of electronic components, often results in the need to make the device reusable, adding use steps and further complications. By transferring the connectivity to a MEMS smart cap on the sharps container, the drug delivery device can remain simple – there is no alteration of the usability due to added electronics. Furthermore, the smart components are kept well away from any drug substance, making their reuse and recycling much easier.
Another benefit of using well-established single-use injection devices is that these are well understood by regulators, whereas questions remain when taking connected drug delivery devices through to approval. Whilst some connected devices are already approved and on the market, these are still a relatively new device category. As a result, there are significant regulatory hurdles to obtaining approval for a connected combination device, which can be reduced with Haselmeier and AARDEX’s smart sharps container solution.
“Haselmeier and AARDEX’s smart MEMS cap is device agnostic and available as an off-the-shelf solution for clinical trials, enabling swift and simple integration into a clinical trial to speed up progress through development and decrease the overall time to market.”
Haselmeier and AARDEX’s smart MEMS cap is device agnostic and available as an off-the-shelf solution for clinical trials, enabling swift and simple integration into a clinical trial to speed up progress through development and decrease the overall time to market. Because the solution does not affect the device, it can be integrated into a study without the need for extensive studies to ensure device compatibility and safety.
The Smart MEMS Cap Solution in Action
To illustrate the simplicity of Haselmeier and AARDEX’s smart MEMS cap solution, consider a specific use case:
- On-site initiation: The patient is briefed on the clinical trial protocol by a researcher and receives a supply of standard disposable injection devices, likely similar to what they are already familiar with, and a sharps container with a smart MEMS cap. To the patient, there are no additional use steps compared with a traditional clinical trial using no connectivity whatsoever – everything is automatic.
- Standard use: The patient returns home and self-administers their medication on a weekly to monthly basis for the duration of the trial. The smart MEMS cap logs the time and date when the patient disposes of their injection devices without the need for further input.
- Follow-up visits: During regularly scheduled follow-up visits to the trial site, researchers can use the data collected from the smart MEMS cap to understand the patient’s adherence behaviour and provide them with data-driven feedback to ensure that they remain adherent.
- Adherence interventions: As the study progresses, researchers can optionally receive real-time adherence data via a digital portal. This data can enable them to proactively intervene if the patient is at risk of becoming nonadherent, potentially preventing discontinuation of treatment.
CONCLUSION
By combining Haselmeier’s drug delivery engineering expertise with AARDEX’s known and proven MEMS Adherence Software, the two companies have developed an elegant solution to the challenge of monitoring adherence during clinical trials. Using a smart MEMS cap to log disposal of standard single-use injection devices in a sharps container, clinical trial researchers can monitor adherence across sites in decentralised trials and identify risks in real time, enabling proactive interventions, without the need for a connected drug delivery device.
The smart MEMS Cap offers a simple and easy-to-integrate solution for pharmaceutical and biotech companies faced with the challenge of longer clinical trials with low retention rates, especially for biologics and rare and orphan drugs. AARDEX has a proven track record in the development of digital solutions for the drug delivery industry and Haselmeier is an established and trusted drug delivery device design partner. By partnering to integrate the smart MEMS cap solution, AARDEX and Haselmeier can help ease progress through clinical trials, decrease time to market and save costs, all without impacting the device or regulatory requirements.
REFERENCE
- Vrijens B, Pironet A, Tousset E, “The Importance of Assessing Drug Exposure and Medication Adherence in Evaluating Investigational Medications: Ensuring Validity and Reliability of Clinical Trial Results”. Pharmaceut Med, 2024, Vol 38(1) pp 9–18.

