To Issue 162
Citation: Kaiser-Kershaw S, Hazrati S, Muenscher S, “Digital Twins – A Rapidly Growing Trend in Healthcare”. ONdrugDelivery, Issue 162 (Jun 2024), pp 44–48.
Sylvia Kaiser-Kershaw, Susanne Hazrati and Sebastian Muenscher examine how digital twinning, based on smart and secure wirelessly connected tags and labels, can transform healthcare processes.
The digitalisation technique known as digital twinning gives physical healthcare-related devices, such as injector pens, metered dose inhalers and prefilled syringes, a unique digital identity that can be tracked in the cloud. Digital twins are created using smart tags and labels, equipped with wireless connectivity, which securely capture, read, control and store data locally on the device and transmit it to the cloud where it can be analysed, stored and shared in real time.
In manufacturing and supply chain management, digital twins can increase the efficiency of everyday operations, including automatic data collection, item-level identification, track and trace, inventory updates and analytics. Digital twins can also deliver benefits at the point of care, whether in a medical facility or the patient’s home, to help with tasks such as product authentication, tracking dosages and monitoring expiration dates to ensure proper use and patient safety.
THE TECHNOLOGIES BEHIND DIGITAL TWINNING
The smart tags and labels that enable digital twinning typically use RAIN radio frequency identification (RFID) and/or near-field communication (NFC) for wireless connectivity. The decision of when to use which technology, either on its own or in combination, largely depends on the use case, reading distance, functionality and the number of items being read.
RAIN RFID, which is a familiar part of automated logistics and inventory tasks, is primarily used for long-range bulk reads. Dedicated RAIN RFID readers can accurately identify over 1,000 items per second at a distance of up to 10 m or more and without line of sight. NFC, on the other hand, which is perhaps best known for enabling contactless payments and as a standard feature in today’s smartphones, is used for individual reads and interactions that happen at close range. A hybrid technology, high-frequency (HF)-NFC, combines vicinity-range applications of up to 1.5 m, using HF readers, and NFC functionality for data exchange at close range.
“Recent advancements include crypto-secure authentication tags, which provide high-level data protection, and smart-sensor tags, which can detect condition changes and events, such as opening detection, pressure or fill level.”
A smart tag, based on RAIN RFID, NFC or a combination of the two, is added to a traditional marking label or embedded in the product itself. The tags are mostly passive, meaning they draw power from the reader and do not require a battery, and can be designed to support a variety of functions, security levels and memory sizes. Recent advancements include crypto-secure authentication tags, which provide high-level data protection, and smart-sensor tags, which can detect condition changes and events, such as opening detection, pressure or fill level. For designs requiring continuous condition monitoring or non-dependency on a mobile phone, battery power is usually necessary.
DIGITAL TWINNING USE CASES FOR RAIN RFID
In manufacturing and the supply chain, digital twinning based on RAIN RFID enhances supply track and trace and inventory management. NXP’s UCODE line of RAIN RFID tag products supports a wide range of customer requirements, from storing the electronic product code to adding user memory, conductive tag-tamper features and authentication.
Smooth Medication Management
The data stored on RAIN RFID labels, such as product name, manufacturer, batch number and expiry date, are read automatically either individually or in bulk using simple handheld or specialty readers and matched with a database. This enables inventory tracking and provides transparencies about medicines that are approaching their expiration date and need to be restocked.
A recent study by Zebra revealed that 74% of UK and US hospital leaders acknowledge that cancellations of procedures or surgeries due to out-of-stock, low-stock or lost supplies are a significant problem. Additionally, 77% of them agree that clinical staff spend too much time searching for supplies when needed, and 75% say it is a challenge to recover all recalled or expired items.1
“By providing precise inventory counts in real time, automated
inventory tracking also helps to detect and reduce drug diversion.”
Containers equipped with RAIN RFID labels can be automatically read at various stations, on the factory floor, in a warehouse or on the hospital ward, for live monitoring and control of the logistic material and product flow. With a read-rate accuracy of up to 100%, products that have been picked and placed are captured automatically, so current inventory levels are consistently stored in a database. By providing precise inventory counts in real time, automated inventory tracking also helps to detect and reduce drug diversion.
A Solution for Prefilled Syringes
Prefilled syringes tend to use materials, including thermoplastic polymers, polypropylene or glass, that can affect the range and read performance of a RAIN RFID tag. Size is a consideration, too, since a small syringe limits space for the RAIN RFID tag and tight curvature can reduce performance. The dielectric properties of medications contained in the syringe can present challenges, too, since water-based active ingredients have a negative impact on radio transmission. Positioning of the RAIN RFID label and its integrated inlay need to reflect the type of liquid and fill level of the syringe.

Figure 1: Cap-Lock label with Integrated UCODE® chip.
Schreiner MediPharm has developed a RAIN RFID label solution for syringes that addresses these issues (Figure 1). Used with a cap adapter, which equalises the diameter differences of the syringe body and cap, the label wraps around the syringe body and the cap adapter. When the syringe is opened, the label’s integrated perforation provides irreversible visual evidence of the event. At the same time, NXP’s label-integrated UCODE tag also records digital evidence of the event upon tag readout, so it is easier to automate inventory control and track syringes based on opening status. The syringe label uses a flexible, yet highly robust, construction suitable for high-speed application, making it suitable for use as part of the normal primary container label process within pharmaceutical production – eliminating the need for application by hand at the point of use. Each label is equipped with a special structure that absorbs mechanical stress and impacts, thereby protecting the integrated inlay from damage and functional failure, so the label can be read reliably from production to final use.
DIGITAL TWINNING USE CASES FOR NFC
NFC helps to support the point of use, either in a medical facility or at home, in what is referred to as the “last patient mile”.
“Automated communication, enabled by NFC, can assist patients interactively in administering their medication, making it easier to adhere to a therapy plan.”
Interactive Patient Assistance
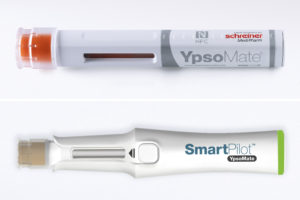
Figure 2: Autoinjector label with ICODE® chip and SmartPilot™ device.
Automated communication, enabled by NFC, can assist patients interactively in administering their medication, making it easier to adhere to a therapy plan (Figure 2). Using NFC technology, Schreiner MediPharm has developed a label for Ypsomed’s YpsoMate disposable autoinjector. The label serves as a communication interface between the injector and the SmartPilot™, a reusable add-on for the device with embedded sensor and identification technology. The smart device can identify and authenticate the medication automatically and check its expiry date. The injection date and time, as well as the delivered dose, are tracked and transmitted to the patient’s smartphone app via Bluetooth Low Energy. The patient is guided interactively through the injection process, assisted in using the autoinjector correctly in real time or informed about inconsistencies – for example, in the event of deviations from the therapy plan or an accidental attempt at a double injection.
Cryptographic Fraud Protection and Web Authentication
The WHO identifies counterfeit drugs as one of the urgent healthcare challenges globally.2 To combat the increasingly serious problem of counterfeit medicines, NFC security tags provide authenticity, integrity or even confidentiality to protect tagged products against falsification, cloning, unauthorised access and other fraud. NXP’s NTAG and ICODE DNA authentication tag chips are equipped with standard-based AES-128 cryptography and can be pre-provisioned with secrets in trusted environments for secure product integration.
NXP’s security-certified NTAG DNA tags feature a secure unique NFC (SUN) authentication message for app-less web authentication, which changes dynamically upon each tap. Only an original tag can generate a valid SUN message and each tagged item can be reliably authenticated. In this way, inspectors, healthcare staff and consumers can verify product authenticity using their smartphones without downloading a special app. Patients can also take advantage of on-demand access to product information and dosage instructions, including videos, helpful digital tools and more.
Medication Authentication with Offline Device
NFC also lets patients verify the authenticity of pharmaceuticals without using a smartphone. A reusable injection device or pen, for example, can be equipped with a small-footprint NFC reader that communicates directly with an NFC tag in or on the medication’s container. The NFC transaction can be used to assure the originality of the consumable at the point of use, even by using an advanced mutual authentication scheme for offline authentication, and can also confirm the type of drug and its batch number, check whether it is within its expiry date or register dosage events.
“Knowing that a medication has its original factory seal intact helps ensure that the product is genuine and has not been modified after leaving the factory.”
First-Opening Detection
Knowing that a medication has its original factory seal intact helps ensure that the product is genuine and has not been modified after leaving the factory. Electronically tamper-evident NFC tags can monitor opening in one of two ways. With a “tamper-loop” tag, the product label or seal is equipped with a conductive loop that, when broken, triggers the NFC tag to irreversibly write a “once opened” status to its memory and sends the status change to the cloud, all when tapped with a smartphone. With a capacitive tamper-proof tag, tamper evidence can be integrated into the product itself – as part of the physical bottle closure, for example. The tag measures capacitance changes, comparing them with preconfigured limits to sense opening, and provides a readout of opening status when tapped with a smartphone. Both types of tamper-evident tags work with web-based authentication without an app.

Figure 3: Autoinjector label with NTAG® TagTamper chip.
NXP’s NFC chips are part of a tamper-evident label Schreiner MediPharm has developed for autoinjectors (Figure 3). The label not only confirms originality and sends a message when first opened but can also be configured to provide additional information, such as product details, demo videos and links to therapy-supporting apps. This type of tamper-evident label can migrate to NXP’s NFC DNA technology so as to add highly secure encryption technology, which enables advanced digital integrity protection. By adding geo-tracker linking, the label-integrated tag can also be used to protect supply-chain integrity by detecting grey-market activities in local markets.
Medication Adherence
Increasingly common chronic conditions, such as diabetes, asthma, chronic obstructive pulmonary disease and arthritis, as well as mental disorders, typically require long-term use of medications. It is not always easy for patients to stay on track with their treatment regimens, especially if they are taking multiple medications for a range of conditions, and many patients struggle with the directions and schedules for proper dosing. The WHO estimates that adherence to chronic medication is only 50%.3
Using an NFC-enabled smartphone to tap an NFC-tagged drug-delivery device, such as an autoinjector or inhaler, can help patients adhere to medication schedules. To help manage asthma treatments, for example, Smart Respiratory Products (London, UK) uses an add-on smart cap that goes over a metered dose inhaler. A connected NXP NFC tag has a wired link to a microcontroller that captures pressure input. Patients can reliably track their inhaler use by pressing the button on the cap to inhale and then tapping the inhaler to a smartphone, which records the exact time of the dosage. Results are made available in a companion app on the NFC smartphone, so the patient knows how many puffs have been taken and when, as well as when the inhaler is running low.
Smartphone apps can be configured to provide other options, too, such as linking to medication guides, usage videos and other helpful information, such as side effects, or establishing daily treatment reminders.
“Having access to real-time data about the frequency and quantity of dosages can help clinical researchers reduce instances of non-adherence and improve the quality and reliability of the data they use in their studies.”
Clinical Trials
Having access to real-time data about the frequency and quantity of dosages can help clinical researchers reduce instances of non-adherence and improve the quality and reliability of the data they use in their studies.
Schreiner MediPharm has developed a Smart Blister Wallet (Figure 4) that can track pills individually as they are removed from their blister cavity. Configured to accept blister packs with up to 64 cavities, the Smart Blister Wallet is a cardboard box equipped with integrated conductive lines and an electronic sensing unit that includes an NTAG tag. When a pill is ejected, an electronic track is broken, triggering the onboard sensor to store the exact time and data at which the pill was removed and the location. Collected sensor data can be read out using a reader or a smartphone with an installed app. Data from the Smart Blister Wallet can be viewed locally on the device or sent to a cloud database for storage and further analysis. Notifications can also help remind patients when their next dose is due, and a message or alert can be sent when doses are missed. Manufacturers can process the Smart Blister Wallet as usual, filling it with standard bifold or multifold blister packs. The package design can also be customised and, thanks to roll-to-roll processes, the level of quality remains consistent, even at higher volumes.

Figure 4: Smart Blister Wallet with NTAG sensing.
CONCLUSION
Digital twins represent an important trend in the digitalisation of healthcare. NXP is at the forefront of this trend, developing advanced security, wireless connectivity, intelligent processing and smart sensing solutions that enable a secure, flexible approach to digital twinning. NXP NFC-RFID solutions enable smart tags, labels and packaging solutions for patient-safe medication and medical devices, such as those provided by Schreiner MediPharm, that optimise healthcare processes, enhance product safety and help avoid medication errors.
REFERENCES
- “Hospital Materials Management Vision Study”. Web Page, Zebra, accessed May 2024.
- “Substandard and falsified medical products”. Web Page, WHO, accessed May 2024.
- “Failure to take prescribed medicine for chronic diseases is a massive, world-wide problem”. WHO, Jul 2003.

