To Issue 177
Citation: Fetz N, “Advancing Lyophilised Drug Delivery with Reunite™: Reconstitution Made Simple”. ONdrugDelivery, Issue 177 (Sep/Oct 2025), pp 26–31.
Dr Nina Fetz introduces SHL Medical‘s Reunite™, the first and only commercialised dual-chamber cartridge, three-step autoinjector featuring the company’s Needle Isolation Technology. With automated reconstitution, Reunite enables reliable self-injection of lyophilised formulations, supporting home-based care models.
In today’s injectable drug landscape, lyophilisation offers both scientific and strategic advantages. Lyophilisation is essential for the safe and effective use of several biologics and other novel therapies that are unstable in liquid form at their desired storage, shelf life and/or use conditions. A lyophilisation step during manufacturing preserves drug stability, extends the shelf life and maintains therapeutic efficacy.
THE EXPANDING ROLE OF LYOPHILISATION IN MODERN DRUG DEVELOPMENT
Today, approximately one-third of US FDA-approved parenteral medications are lyophilised products,1 and the global lyophilised drug market is estimated to grow from US$371 billion (£274 billion) in 2025 to $683 billion by 20322 – a trend that reflects the continuing importance of lyophilisation in modern pharmaceutical development.
From a financial perspective, although they often involve higher initial manufacturing costs, lyophilised drug products offer long-term advantages.3 Many liquid formulations depend on cold chain logistics, which account for up to 20% of pharmaceutical logistics costs and exceeded $12.6 billion globally in 2016,4 creating ongoing operational burden and inventory risks (for example, product loss due to temperature excursions). Lyophilised formulations can reduce these dependencies, enable more flexible storage and distribution, lower long-term costs and minimise supply chain vulnerabilities. This also brings sustainability advantages, cutting down on energy-intensive cold chain requirements and minimising the carbon impact of distribution.
CHALLENGES IN LYOPHILISED DRUG DELIVERY
Despite this growth and the strategic advantages, the delivery of lyophilised therapies has long been associated with significant usability challenges. Lyophilised products are traditionally presented in vial kits (Figure 1), often requiring separately packaged diluent and a transfer syringe for manual reconstitution. These vial kits typically involve many separate user steps, sometimes 12–15 or more.
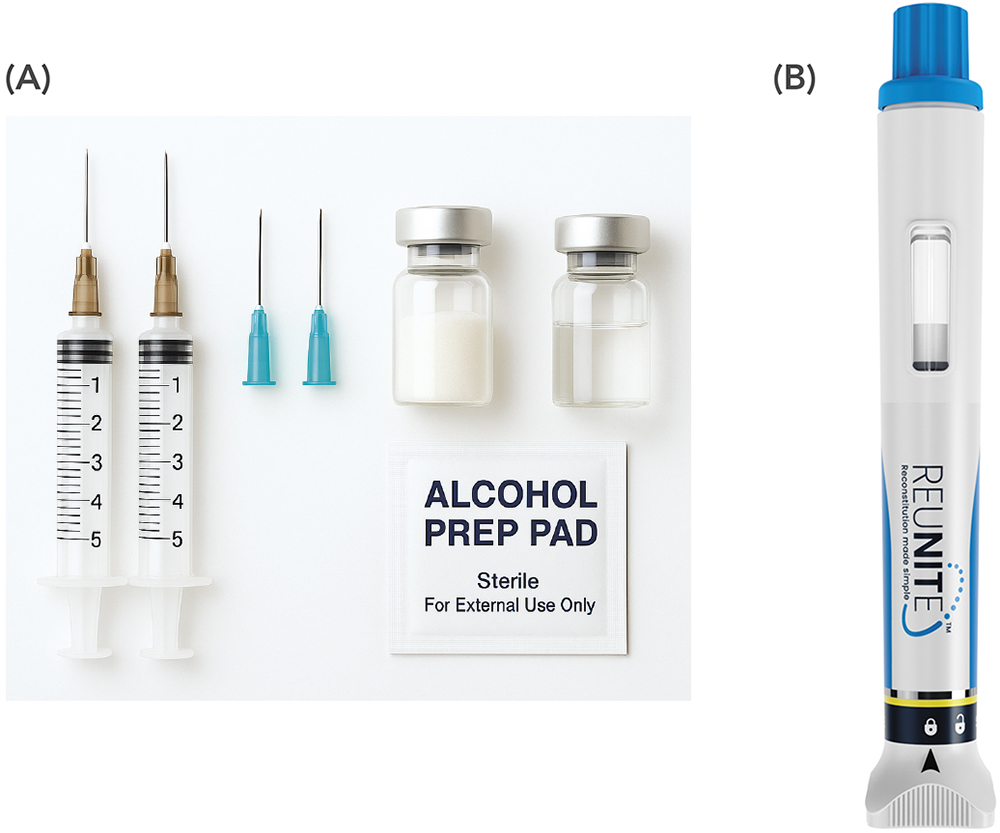
Figure 1: (A) Traditional vial kit for lyophilised products. (B) The new Reunite™ autoinjector.
The process of reconstituting a drug requires a high degree of knowledge, skill and technique to enable safe preparation and sterile injection. To correctly perform this long sequence, it can be complex, taxing and time-consuming, all of which increase the risk of user error. For these reasons, kits represent a practical barrier to self-administration, as patients may require additional training and healthcare provider (HCP) support.
Recent innovations aim to address these challenges by streamlining preparation. For example, prefilled diluent syringes/vial kits and similar solutions reduce the total number of preparation steps, but do not fully eliminate complexity, as manual reconstitution and handling of multiple components are still required in most applications. In parallel, regulatory bodies are placing growing emphasis on the performance of drug-device combination products, with particular focus on accurate dose delivery,5,6 protection from sharps injury7 and post-reconstitution stability.8
To fully realise the potential of next-generation lyophilised therapies, the industry should seek to move beyond traditional multistep kits and manual HCP administration. This shift calls for innovative, patient-centred solutions that are easy to use, support accurate and reliable self-injection, and enable home-based care.
INTRODUCING REUNITE™, AN INTEGRATED APPROACH TO LYOPHILISED DRUG DELIVERY
SHL Medical has responded to these challenges with an integrated system approach, reimagining lyophilised drug delivery as a simple, safe and patient-centred experience.
This vision is realised in Reunite™ (Figure 1), the first autoinjector that integrates reconstitution and administration of lyophilised formulations in one device featuring SHL Medical’s Needle Isolation Technology (NIT®).
“REUNITE BECAME THE FIRST AUTOINJECTOR OF ITS KIND TO RECEIVE MARKETING AUTHORISATION FOR THE SUBCUTANEOUS DELIVERY OF A LYOPHILISED MONOCLONAL ANTIBODY TARGETING THE IL-31 SIGNALLING PATHWAY.”
Reunite became the first autoinjector of its kind to receive marketing authorisation (approved in the US in 2024 and subsequently in the EU, UK and Switzerland in 2025) for the subcutaneous delivery of a lyophilised monoclonal antibody targeting the IL-31 signalling pathway, a central mediator of chronic itch in prurigo nodularis and atopic dermatitis.
Reunite is built around a dual-chamber cartridge (DCC) and uses SHL Medical’s proven NIT technology to enable automated reconstitution and injection. As a result, Reunite greatly reduces the total number of user steps, from 12 to 15 required to manually reconstitute down to just three: Unlock, Twist, Push. The autoinjector greatly simplifies the reconstitution process, removing the need for the precise manual technique required when using a traditional vial kit. Its enhanced usability features – which include a locking mechanism, needle safety shield and reduced number of steps – collectively minimise the risk of user error. This ensures more reliable self-administration and therefore greater patient independence. The unique internal design of the Reunite autoinjector is detailed in the next sections.
Inside Reunite: DCC and NIT
At the core of Reunite is the DCC that functions as the primary container, securely housing the lyophilised drug and its diluent in separate compartments (Figure 2). Upon user activation, a single spring mechanism drives an automated sequence that initiates reconstitution by mixing the contents of both chambers. The lower chamber contains the diluent, and the upper chamber contains the powder. When activated, the diluent moves through a bypass into the upper chamber. This design eliminates manual reconstitution steps, minimises cognitive demand, supports fixed-dose preparation and delivers consistent mixing prior to administration.
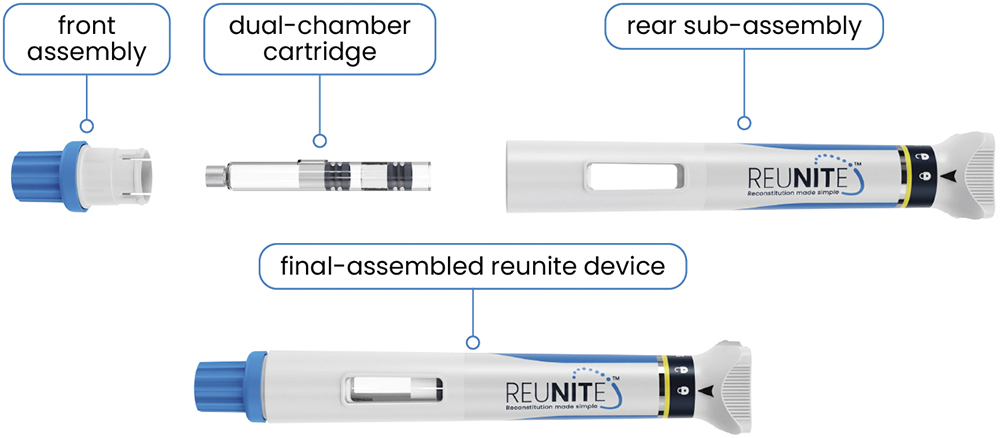
Figure 2: DCC containing lyophilised drug and diluent in separate compartments, enabling automated reconstitution at the point of use.
“IN 2019, THE NIT AUTOINJECTOR WAS SELECTED AS THE GOLD WINNER OF THE MEDICAL DESIGN EXCELLENCE AWARD IN THE DRUG DELIVERY AND COMBINATION PRODUCTS CATEGORY.”
To complete the delivery sequence, Reunite features SHL Medical’s proprietary NIT technology, which enables safe, automated needle attachment while maintaining full needle concealment before and after use (Figure 3). Integrated into the cap, the sterile cannula pierces the cartridge septum automatically when the cap is twisted off, opening the fluid path without requiring needle attachment by the user, eliminating the risk of needlestick injury and contamination.9 NIT also provides the drug developer the option to change cannula gauge and length, without implication for long-term drug stability.
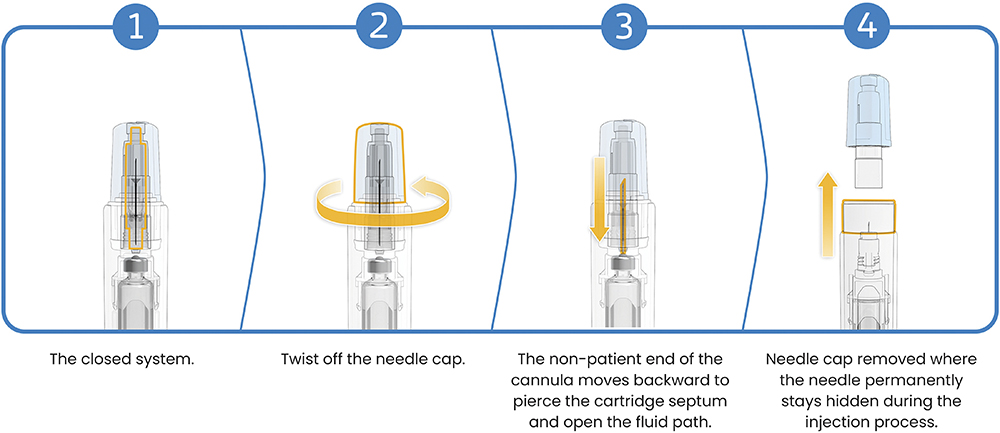
Figure 3: NIT keeps the needle concealed before and after use, enabling safe, automated needle engagement and drug delivery.
The first NIT autoinjector was developed for a suspension formulation for type 2 diabetes. First approved in 2017 and commercialised in 2018, it has delivered millions of doses to patients worldwide. In 2019, the NIT autoinjector was selected as the Gold Winner of the Medical Design Excellence Award in the drug delivery and combination products category.
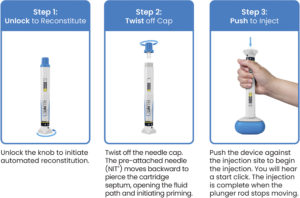
Figure 4. Reunite™ enables a simple three-step user experience: unlock, twist, push. In parallel, the device performs its own drug delivery sequence: reconstitute, prime, inject.
Reconstitution Made Simple: The Reunite User Experience
By integrating a DCC and NIT into a single device, Reunite transforms the traditionally complex process of lyophilised drug delivery into a simple three-step experience (Figure 4). Upon unlocking the knob (Unlock), the user initiates an automated sequence: the internal spring mechanism drives reconstitution as the diluent mixes with the lyophilised drug. By twisting the cap in a second step (Twist), the user initiates priming and the sterile, pre-attached cannula engages to establish the fluid path. As a final step, the user presses the device against the skin to activate the injection (Push).
Each step has been carefully engineered for simplicity and reliability. There are no separate components to assemble, no manual mixing steps and no exposed needles. Audible feedback at the start of the injection guides the user through the different stages, providing clear reassurance and supporting proper handling.
PARTNERING WITH LYOTECH FOR STREAMLINED PROCESSES
Just as Reunite integrates reconstitution and delivery within a single device, the successful commercialisation of lyophilised therapies depends on the integration between formulation, filling and final assembly processes. Recognising this, SHL Medical has collaborated with leading CDMOs offering lyo-liquid formulation and filling, including Lyophilization Technology. Building on the company’s extensive experience in process development for lyophilised products and DCC filling of lyo-liquid combinations, the partnership of SHL Medical with Lyophilization Technology, Inc accelerates the path from formulation to launch while reducing development risks.
“REUNITE OFFERS PHARMACEUTICAL COMPANIES A STRATEGIC PLATFORM TO UNLOCK THE POTENTIAL OF LYOPHILISED THERAPIES BY COMBINING DRUG STABILITY WITH SIMPLIFIED SELF-ADMINISTRATION AND INTEGRATED COMMERCIALISATION PATHWAYS.”
THE STRATEGIC VALUE OF REUNITE
Reunite offers pharmaceutical companies a strategic platform to unlock the potential of lyophilised therapies by combining drug stability with simplified self-administration and integrated commercialisation pathways. Built around a DCC and featuring SHL Medical’s proprietary NIT, Reunite is the first commercialised dual-chamber, three-step autoinjector to support automated reconstitution and injection in a single device. It delivers value across drug formulation, patient experience, supply chain and time to market. Key advantages include:
- Expanding Access to Lyophilised Therapies: Reunite removes historical barriers to the use of lyophilised drugs, such as the need for multicomponent vial kits, manual reconstitution and clinic-based administration, thus driving the commercial viability of therapies that require lyophilisation for stability.
- Empowering Patient-Centric Self-Administration: Designed as a three-step autoinjector, Reunite delivers a safe and intuitive user experience. The integrated design reduces total handling steps, conceals the needle, and provides visual and audible cues, empowering patients to self-administer their medication with confidence and minimal training at home.
- Enhancing Operational Efficiency and Supply Chain Resilience: Lyophilised stability reduces dependence on cold chain logistics, minimises inventory risks and strengthens the supply chain.
- Accelerating Development and Reducing Risks: SHL Medical’s expertise in device development and its partnership with LyoTech, an expert in process development for filling lyophilised drugs helps pharmaceutical partners to streamline development timelines, reduce risks and accelerate time to market.
Together, these capabilities position SHL Medical as a strategic partner for pharmaceutical companies seeking to deliver novel therapies safely, efficiently and with a greater focus on the patient experience, making Reunite the platform of choice for lyophilised drug delivery.
REFERENCES
- Kumar S et al, “Application of lyophilization in pharmaceutical injectable formulations: An industry and regulatory perspective”. J Drug Deliv Sci Technol, 2024, Vol 100, art 106089.
- “Lyophilized drugs market analysis and forecast: 2025-2013”. Coherent Market Insights, Jul 2025.
- Cullen S et al, “Technical transfer and commercialisation of lyophilised biopharmaceuticals – application of lyophiliser characterisation and comparability”. AAPS Open, 2022, Vol 8, art 14.
- “Cold Chain Logistics Report”. Pharmaceutical Commerce, 2016.
- “Draft Guidance for Industry: Essential Drug Delivery Outputs for Devices Intended to Deliver Drugs and Biological Products”. US FDA, Jun 2024.
- “Suitability of Graduation of Delivery Devices for Liquid Dosage Forms”. EMA, Feb 2005.
- “Guidance Document: Medical Devices with Sharps Injury Prevention Features – Guidance for Industry and FDA Staff”. US FDA, Aug 2005.
- “Guidance for Industry Q1A(R2): Stability Testing of Drug Substances and Drug Products”. US FDA, Nov 2003.
- Calderwood G, Ganea R, Fuensalida Pantig GR, “Enlarging the Volume of Autoinjectors: Traversing Injection Boundaries”. ONdrugDelivery, Issue 138 (Oct 2022), pp 26–32.

