To Issue 144
Citation: Hellbardt S, “Interview – De-Risking Combination Product Registration for Semi-Solids with Aptar Pharma’s Airless+ System”. ONdrugDelivery, Issue 144 (Mar/Apr 2023), pp 19–22.
Stefan Hellbardt discusses the increasing demand for airless systems in the dermal drug delivery market, the advantages of these systems and Aptar Pharma’s own offering in this space, including its Airless+ packaging system and Extended Support services.
Q What is airless pharmaceutical packaging?
A Airless packaging systems are an increasingly prevalent form of dermal formulation packaging used by pharmaceutical and consumer product companies; their key functional property being that they protect their contents from exposure to air. This is important for many pharmaceutical formulations that would otherwise become unstable and degrade or break down.
At Aptar Pharma, our focus in this area has been on developing our Airless+ systems for semi-solid pharmaceutical formulations, such as lotions and gels. For these applications, in addition to protecting the formulation from exposure to air, it is also important that the packaging system can deliver a precise dose of formulation with a clean and convenient user experience. Aptar Pharma was the first company to create an airless dermal drug delivery system specifically designed to meet the requirements of the pharmaceutical industry in highly regulated markets, such as Europe, the US and China.
At Aptar Pharma, we have designed our Airless+ systems using medical-grade resins in order to meet the requirements of prescription medicines. The need for the enhanced product evaluation and documentation that is able to satisfy regulators is increasingly important – it is a critical part of drug-device combination filings for customers.
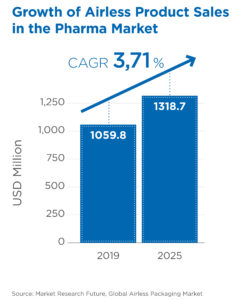
Figure 1: Airless product sales are expected to grow by over 3.7% until 2025 (CAGR). Source: Market Research Future, Global Airless Packaging Market.
Q What are the trends in the airless pharmaceutical packaging market?
A Overall, we have seen a growing interest, from both our customers and their end consumers, in premium packaging solutions that provide enhanced dermal product protection. The global airless pharma packaging market is expected to reach US$1.3 billion (£1.1 billion) by 2025, with a 2019–2025 compound annual growth rate (CAGR) of 3.7% (Figure 1).1 This growth is expected to be the highest in Europe, North America and Asia Pacific, which currently account for more than 75% of the global airless pharmaceutical packaging market. Historically, most airless packaging applications have been for conventional small-molecule drugs and macromolecules, but we have started to see an increase in new product applications for novel treatments, such as microbiome therapies.
Q Are you seeing increased demand for Aptar Pharma’s Airless+ packaging systems?
A Our Airless+ dermal delivery systems are absolutely growing in popularity, and we have identified several causes for this increased demand. The main advantage of airless devices is that they provide protection for the product formulation, which can be quite expensive; by eliminating any contact with air, it allows even air-sensitive products to be packaged in a multidose dispensing system that can deliver reliable dosing over time. Additionally, our Airless+ systems offer a premium appearance and potentially extend the in-use period compared with more commonly used tube packaging.
For the product manufacturer, our Airless dermal delivery systems are fillable on most current filling lines, which makes manufacturing more cost efficient and flexible to implement. Customers who fill their products into Airless+ packaging systems value product protection, flexible and easy manufacturing implementation, and product differentiation.
Q Is the growing demand for airless packaging systems also influenced by end users?
A Yes, patients and users have also started to discover the benefits of airless packaging systems, citing features such as ease of use and clean dose delivery as attractive benefits (Figure 2). Airless packaging can also extend the lifespan of a product, and the efficiency of the dispensing system means there is minimal waste of valuable product formulation. Another functional benefit of airless packaging systems is that their leak-resistant properties improve their portability, allowing them to be safely carried in a handbag or backpack. Patients can self-administer a precise dose of semi-solid formulation with 360° functionality; the dispenser can be oriented in virtually any direction when used.
Figure 2: Precise dosing, mess-free application and convenient handling are key advantages that patients appreciate in dermal administration.
“Our Airless+ systems offer a premium appearance and potentially extend the in-use period compared with more commonly used tube packaging.”
Easy application to the last dose is a key feature of Aptar Pharma’s Airless+ dispensers. Patients don’t have to undergo the frustration of squeezing the last drops of their potentially costly cream or lotion from a conventional foil tube. Instead, the easily emptied Airless+ packaging system can be directly recycled into existing recycling streams without any additional separation of materials. Patients prefer our Airless+ systems because they offer both convenience and functionality.
Q There is a growing demand for more sustainable products and packaging. How is Aptar Pharma answering this demand with its device and service offering?
A In a 2022 Aptar Pharma survey conducted with 840 German, French and American participants, 77% of respondents indicated that it was important or very important that the products they buy can be recycled. Additionally, 60% of respondents noted that they consider the recyclability of products when making purchase decisions, while 70% of respondents are willing to pay more for a product that they can recycle. Our Airless+ systems are designed to eliminate the inclusion of disruptive materials and metal parts. Instead, they only use polyolefin resin materials, which increases their recyclability – noted by their “Class AAA” and 96–98% “excellent recyclability” rating by Institut cyclos-HTP (Aachen, Germany). Our Airless+ systems provide the functional efficiency and sustainability the market is currently demanding of packaging solutions.
Q How do the increasing demands of regulatory requirements impact the Aptar Pharma Airless+ product line?
A As one of the world’s first developers of airless packaging systems for medical and consumer markets around the globe, we have an unrivalled understanding of the regulatory requirements affecting these products. In the US, they are considered drug-device combination products, and, in the EU, they are regulated under the Medical Devices Regulation. In both regions, we have a great deal of experience supporting customers with regulatory requirements. In China, we have found the Chinese regulatory requirements to be a little different, but we have established the needed China-FDA drug master files and are supporting our customers with the testing and respective regulatory documentation. They require some special tests and documentation that need to be filed in a dossier with the Chinese regulatory agency.
Aptar Pharma has a long history of successfully providing customers with regulatory support in major markets. Especially for smaller companies, who may not have a large regulatory team, this support can be essential. With our larger customers, our regulatory expertise makes life significantly easier for them. Our customers know that Aptar Pharma has the experience and expertise to get them through the increasingly complex regulatory process, even for the latest drug-device combination product regulations, from the device perspective. Our original Airless+ systems have numerous market references and a proven track record of customer product approvals from major regulatory bodies. This regulatory knowledge helps us de-risk new drug development projects for our customers almost anywhere in the world.
“Our recent dossier filing in China represents a massive opportunity for Aptar Pharma to serve customers in a huge new market.”
Q How does Aptar Pharma support the customer journey to market for products when using Airless+ systems?
A Aptar Pharma does more than just design and manufacture Airless+ devices. We offer customers a full range of Airless+ support services through our Extended Support (ES) offering; helping the customer with device selection and customisation to ensure that they have the right technology solution for their application. Our ES offering also includes access to our specialised analytical development and lab testing services. We can generate Airless+ device extractables data according to relevant USP/ISO requirements. Once the customer has the right device, we can offer specialised formulation development services to ensure that the formulation is fully optimised for the specific device. Finally, as I mentioned previously, we provide comprehensive regulatory support for customer products using our Airless+ systems.
Q How has the combination of Aptar Pharma devices, regulatory support and ES translated into success for your customers?
A While there are a number of airless packaging providers out there, Aptar Pharma’s offering is different in that we can offer a full, integrated package of services. Our customers know that we have proven successes supporting customer approvals using our Airless+ systems with a range of regulators. We have also started to create dossiers and drug master files with relevant authorities to accelerate the process further. Aptar Pharma’s airless systems have been used in more than 50 commercially marketed drug products.
With our ES offering, we can provide analytical and formulation development services around all Aptar Pharma devices. Customers have used Airless+ drug delivery systems in their drug market applications in the US, EU and China. Aptar Pharma contributes to product drug master files and dossiers specific to the Chinese regulatory requirements for drug product submissions. With Aptar Pharma’s Airless+ dermal drug delivery systems, customers know that they don’t have to manage complex drug-device combination product submissions on their own.
Q How is the Chinese market different from other markets and why is this market important for Aptar Pharma and its customers?
A Aptar Pharma has had success in the EU and US markets with its airless and Airless+ packaging systems for some time. Our recent dossier filing in China represents a massive opportunity for Aptar Pharma to serve customers in a huge new market. With the Chinese topical dermal drug market expected to reach $6.7 billion by 2030, with a 2019–2030 CAGR of 7%,2 China represents the largest market in the Asia-Pacific region. This large population will continue to have high demand for both prescription and consumer dermal products that require protective and precise airless packaging systems. This is as important for Aptar Pharma as it is for our customers wanting to launch differentiated products in this expanding market.
One challenge in China is that the market regulations are different from those in Western markets in certain ways, but we’ve leveraged our experience as a global device manufacturer to adapt to their requirements. We’ve created a market-specific offering for our Airless+ packaging system exclusively for the Chinese market, called Airless+ CS. We’ve also started to move towards creating independent device dossiers that can be referenced in future drug submissions using Airless+ CS dispensing systems to accelerate the process and make submissions in China easier for our customers. Aptar Pharma retains ownership of the dossier, which can then be applied to additional customer submissions directly. These are all huge steps forward for us in a rapidly growing market.

Figure 3: Dermal drug delivery is an important area of focus for Aptar Pharma with its Airless+ packaging systems.
Q What future do you see for airless products and how will Aptar Pharma adapt to this future?
A Aptar Pharma’s Airless+ packaging systems (Figure 3) continue to be an important area of focus for us. With increasing demand for semi-solid dermal formulations and an increasing need for secure delivery systems that protect the product, we’re continuing to invest in optimising our Airless+ drug delivery devices for regional market requirements. We are also continuing to improve the support services available to customers throughout their regulatory journey via our ES offering. We will continue to assess adding new product features, such as child-resistant senior-friendly and digital health functionality, to enhance our product lines. We will also assess local production and sourcing initiatives to be closer to our customers and provide greater security of supply. Lastly, all our products will include sustainability initiatives that lead to technologies and services that have a reduced impact on the environment while still providing elite device performance. We expect that our Airless+ systems will remain at the forefront of functionality and sustainability in airless dermal drug delivery.
To learn more about Airless+ dermal drug delivery systems, visit:
www.aptar.com/products/pharmaceutical/airless-dermal-drug-delivery-system
REFERENCES
- “Airless Packaging Market Outlook and Opportunities in Grooming Regions with Forecast 2027”. Market Research Future, Dec 2019.
- “Topical Drug Delivery Market Report 2020–2030”. Visiongain, Oct 2020.

