Citation: Encinas JL, Ecenarro S, “Quali-V Extra Dry: A Novel Capsule For Delivering Hygroscopic Pharmaceutical Drugs”. ONdrugDelivery Magazine, Issue 99 (Aug 2019), pp 16-18.
Jose Luis Encinas and Susana Ecenarro explore how the use of Quali-V® Extra Dry capsules can help improve product stability and, crucially, lead to efficiencies and savings in drug product manufacture.
A fundamental requirement in drug formulation is that the API remains stable under specific ICH environmental conditions and in the finished dosage form until the end of its shelf life to ensure efficacy and patient safety. The physical and chemical properties of pharmaceutical solids are critically dependent on the presence of water/moisture, e.g. during compaction, stability, storage, and processing into formulation and final product packaging. Many APIs, as well as excipients, are moisture sensitive and/or hygroscopic in nature and need to be protected as moisture can have a possible negative effect on their potency or strength, result in chemical degradation and/or polymorph forms, and could also potentially affect capsule characteristics.
“Particle aggregation due to moisture affects the emitted dose – that is, the powder released from the capsule and device – as well as the amount of drug that reaches and is deposited in the lungs.”
Examples of current APIs in the market that are moisture-sensitive include pancreatin, omeprazole and lansoprazole (proton pump inhibitors), tiotropium bromide (inhalable powder), ranitidine, losartan, enalapril and dabigatran. Some common excipients that also fit into this category include PEG (low molecular weight), glycerine fatty acid esters or medium chain fatty acid triglycerides, sorbitol, maltodextrin, citric acid, microcrystalline cellulose (MCC), polyvinylpyrrolidone (PVP), croscarmellose sodium, sodium chloride, sodium sulphate, ammonium sulphate, amines and calcium chloride.
Inhalable powders are also extremely sensitive to moisture, as aerosolisation properties and drug delivery performance can be greatly impacted by the amount of moisture present in the formulation. Particle aggregation due to moisture affects the emitted dose – that is, the powder released from the capsule and device – as well as the amount of drug that reaches and is deposited in the lungs.
The Quali-V® Extra Dry capsules developed by Qualicaps are specifically designed for use in administering these moisture-sensitive or hygroscopic formulations, both in solid oral and inhaled delivery forms, as the capsule moisture content has been reduced to 2-3.5%, from the standard HPMC capsule of 4-6%.
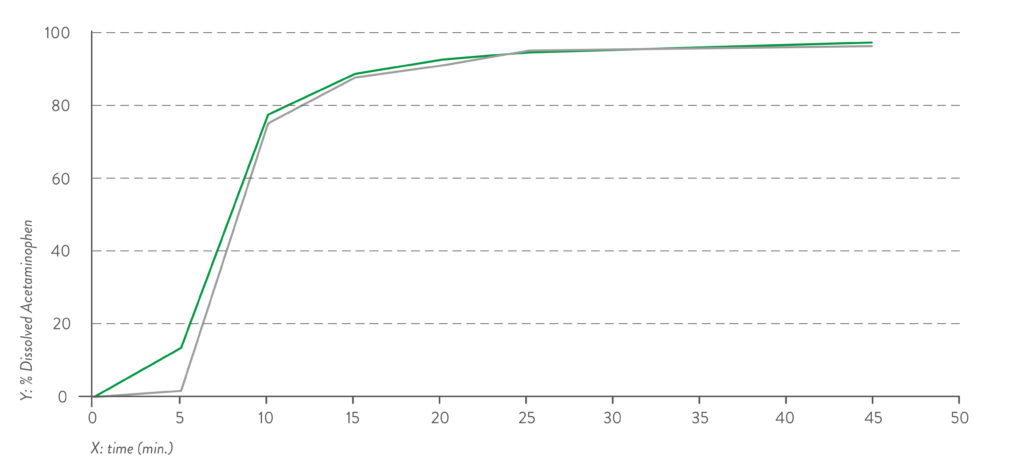
Figure 1: Dissolution profile for pH 1.2 capsule fill formulation – acetaminophen.
CHARACTERISTICS OF QUALI-V® EXTRA DRY
Quali-V® Extra Dry capsules are based on Qualicaps standard Quali-V® hydroxypropyl methylcellulose (HPMC) capsules, developed to respond to the growing market demand for moisture-sensitive and hygroscopic drug delivery. The primary difference is found in the manufacturing process. Traditional HPMC capsules are manufactured to obtain a final moisture content of 4-6% whereas, in order reduce this amount by almost half, the production process for Quali-V® Extra Dry was adapted to incorporate an extra drying phase – but without compromising the capsule’s physical or mechanical properties.
Therefore, like Quali-V®, they are made from plant-based ingredients, preservative free, chemically inert and do not undergo crosslinking reactions.
Quali-V® Extra Dry capsules are also equivalent in their dissolution profile to Quali-V®, which complies with the USP dissolution test requirements of >80% dissolved API at 45 minutes (Figures 1 and 2).
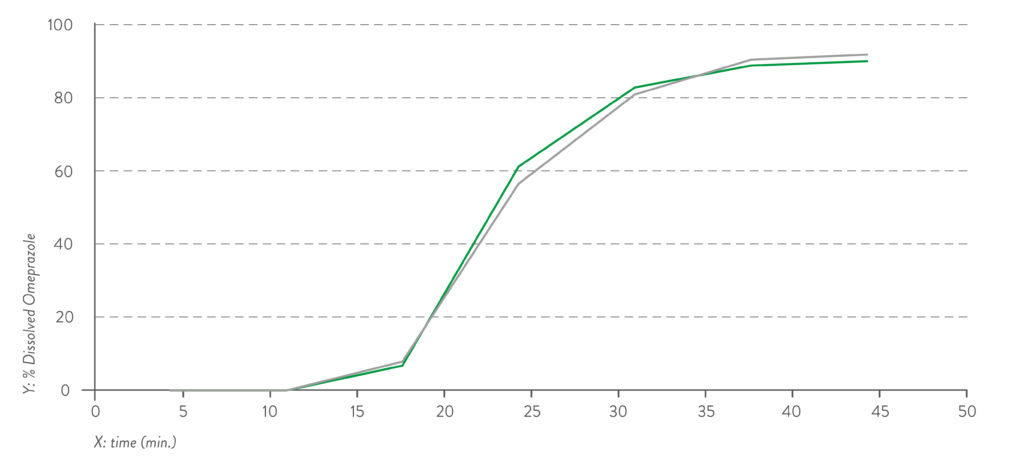
Figure 2: Dissolution profile for pH 6.8 capsule fill formulation – omeprazole pellets.
Quali-V® Extra Dry capsules maintain their physical robustness and present minimal brittleness when exposed to ambient temperature conditions and low relative humidity (RH: 22%), despite their reduced moisture content (Figure 3).
Quali-V® Extra Dry capsules preserve their moisture within the low range of 2-3.5% within ambient conditions from 15% RH to 25% RH – room conditions for low-moisture filling operations (Figure 4).
An important phase of the capsule development process is testing runability for commercial production scale. As such, Qualicaps has also tested Quali-V® Extra Dry capsules on several principal capsule highspeed filling machines – from manufacturers such as MG2, Bosch and IMA – in which they have demonstrated solid performance.
BENEFITS OF QUALI-V® EXTRA DRY IN THE FILLING PROCESS
The typical encapsulation process for moisture-sensitive drug products involves the feeding of the API formulation and empty capsules separately into the filling machine in a controlled environment. API moisture content, as well as filling room conditions, can be set precisely by the manufacturer but capsule moisture will be present throughout the process, during which some interaction with the API and excipients may occur.
Normally the final stage is drying the filled capsule – that is, moisture content is reduced from the final dosage form after the filling process takes place. As removing the moisture from the filled capsules may take up to several hours (some real examples include 6-8 hours, as well as 12-14 hours for different drug products), the drying step becomes a bottleneck in terms of timing within the drug manufacturing process – decreasing the overall yield.
As a result, moisture removal via drying the final dosage form has two principal disadvantages: firstly, there is a certain timeframe in which moisture from the capsule can interact with the moisturesensitive drug formulation and, secondly, drying becomes a time-consuming part of the process with a low added value.
During the runability tests previously mentioned, Qualicaps simulated real conditions of the filling process for moisture-sensitive drug products in order to study the behaviour and performance of Quali-V® Extra Dry capsules. By using these capsules (instead of traditional HPMC), the drying process can be avoided or at least significantly reduced. This means potential savings in total production time, as well as in equipment, production staff and utilities. The capsules also lead to the important benefit of limiting the exposure of the drug formulation (moisture-sensitive or hygroscopic API and/or excipients) to the moisture content present in the capsule.
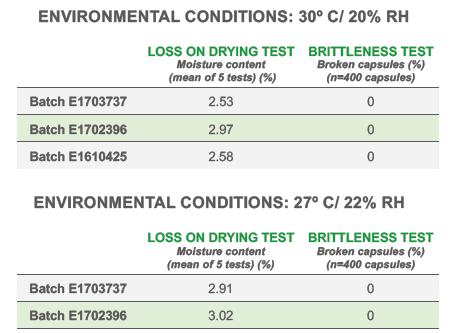
Figure 3: Capsule brittleness tests.
PACKAGING, HANDLING AND STORAGE INDICATIONS
Produced under specific conditions, Quali-V® Extra Dry capsules are packaged in heat-sealed, moisture-proof aluminium liner bags to ensure the specified moisture content for 18 months – the shelf life of the empty capsules (a three-year long-term stability study is ongoing).
To prevent variability in shell moisture content, capsules should always be stored within the recommended temperature range – between 15°C and 30°C (59°F and 86°F). Maintaining the capsules within the liner bag (without perforations) safeguards them from both light degradation and moisture content changes, regardless of ambient humidity.
The conditions in areas where capsules could be exposed to air can affect the defined properties and/or machinability of the Quali-V® Extra Dry capsule. The ideal conditions for capsule filling (to maintain the moisture content of Quali-V® Extra Dry within the specified range of 2-3.5%) are established as follows:
- A temperature between 20°C and 30°C with a recommended set point of 25 ±2°C
- A relative humidity between 15% and 25% with a recommended set point of 20% ±2%.
The above requirement for handling during the filling and packaging process is the only one specific to Quali-V® Extra Dry.
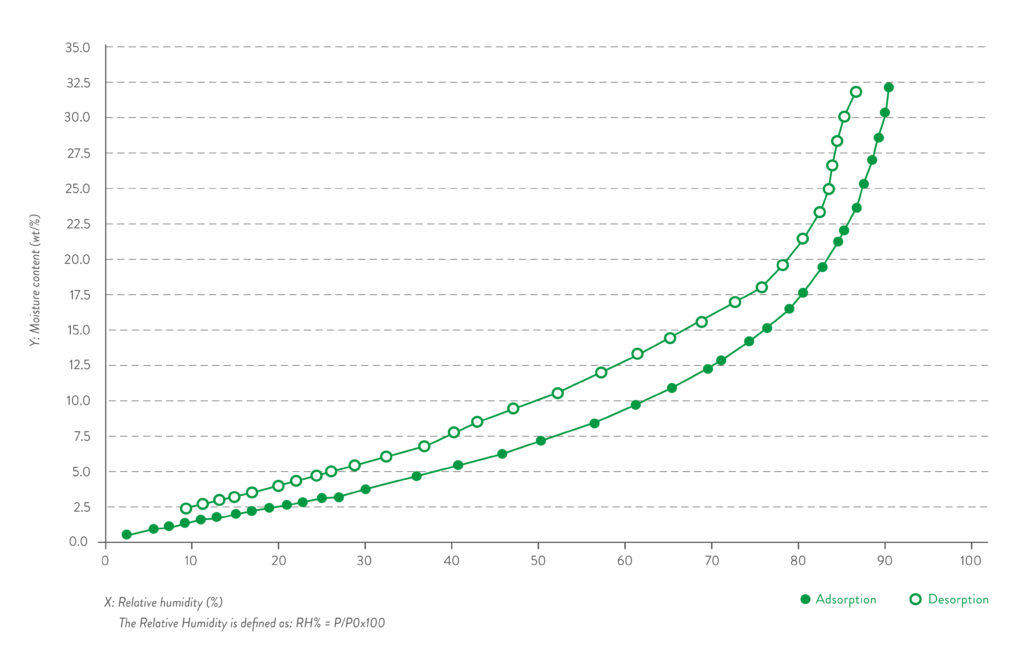
Figure 4: Water vapour absorption/desorption isotherm curve.
CONCLUSION
Qualicaps developed the Quali-V® Extra Dry capsule to address the needs of the pharmaceutical industry as moisture-sensitive and hygroscopic drug formulations are increasing both in the marketplace and in R&D. While there is a current solution for this type of drug product, it depends on the manufacturing process itself, by introducing a drying stage after the capsule filling process. This unfortunately does not address the moisture content inherent in the capsule shell when it comes into contact with the fill – and can also negatively affect overall production yields.
Employing Quali-V® Extra Dry capsules instead of traditional HPMC capsules reduces both of these potential issues – potentially improving product stability as well as leading to efficiencies and savings in drug product manufacture.


